Application of combination of APOE inhibitor and PD-1 monoclonal antibody in preparation of medicine for treating gastrointestinal tumors
A technology of digestive tract tumor and PD-1, applied in the field of medicine, can solve the problem of low effective rate and achieve the effect of improving the effective rate of treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Establishment of wild-type mouse C57BL / 6 subcutaneous liver cancer, intestinal cancer, gastric cancer xenograft model
[0017] C57BL / 6 mice (purchased from Weitong Lihua), 4 weeks old, cell lines (MC38-intestinal cancer, Hepa1-6-liver cancer, MFC-gastric cancer, all purchased from Shanghai Institute of Cell Biology, Chinese Academy of Sciences), using Subcutaneous liver cancer, intestinal cancer, and gastric cancer xenograft models were established by injection in the groin. The specific method is as follows: select cells with good growth status (MC38-intestinal cancer, Hepa1-6-liver cancer or MFC-gastric cancer cells), pour out the culture medium in the bottle, add 2ml of D-Hanks solution, shake it gently, pour it into Add 0.25% trypsin and O.02% EDTA mixed (1:1) 3ml of digestion solution into the bottle, gently shake the culture bottle to make the digestion solution flow over all cell surfaces, and digest at room temperature 25°C for 3 minutes. Observe under an inver...
Embodiment 2
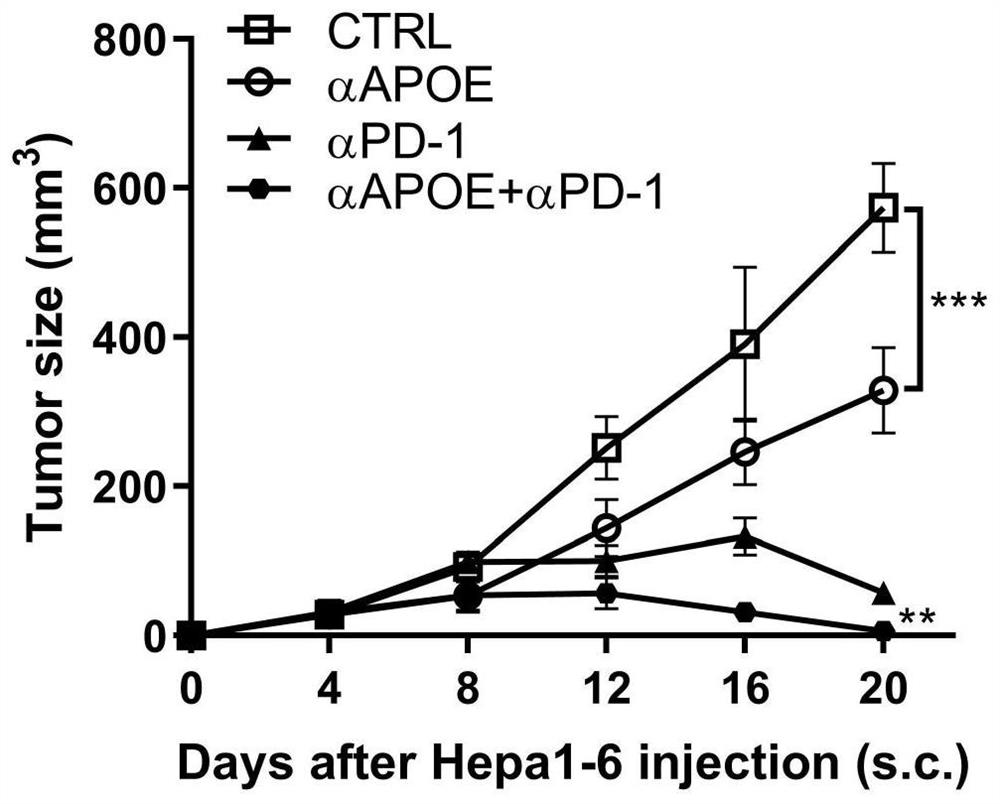
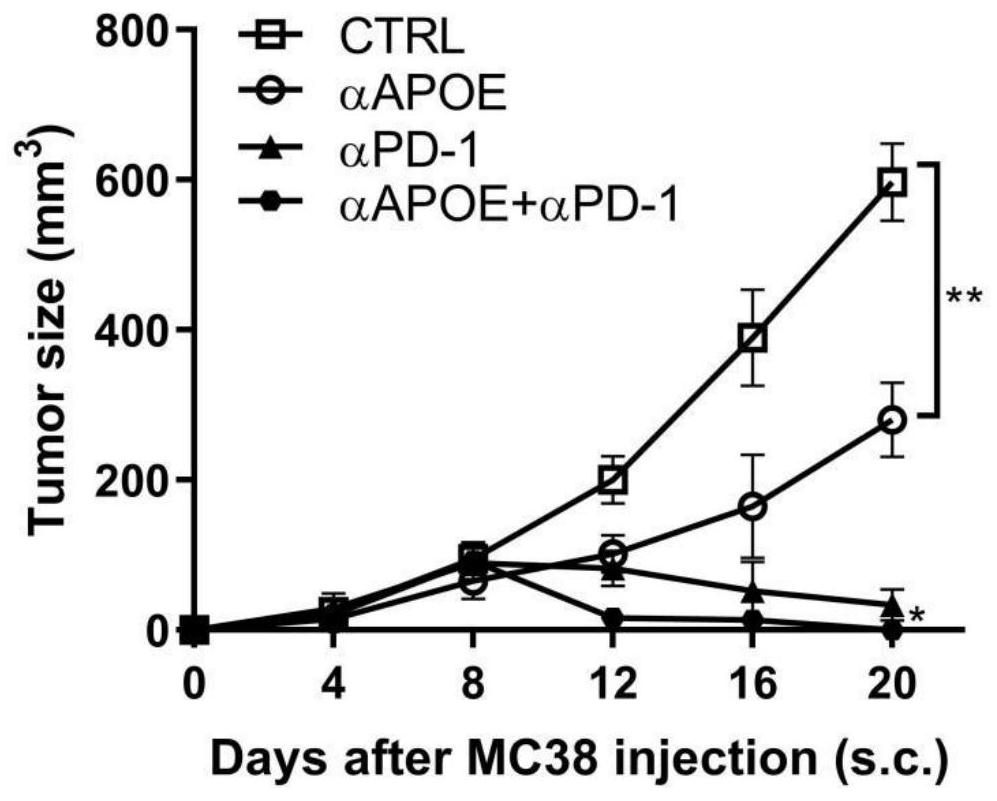
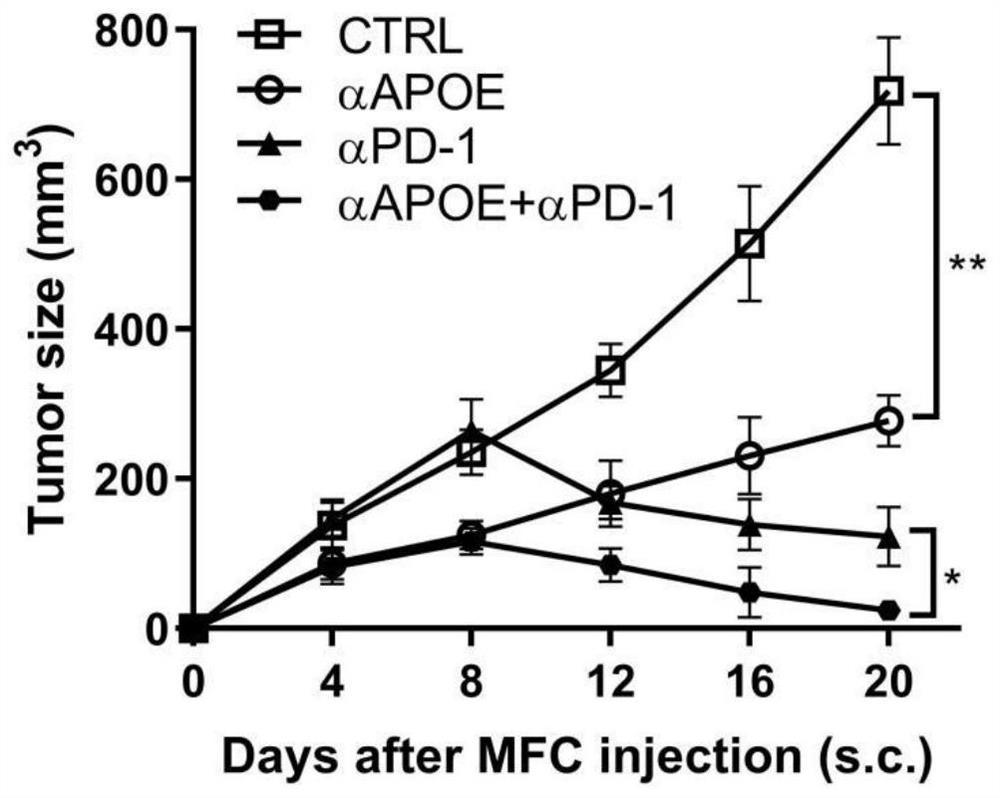
[0019] Combination of APOE inhibitor and PD-1 monoclonal antibody in the treatment of gastrointestinal tumors
[0020] 1. Take liver cancer transplanted tumor model mice and divide them into four groups, which are set as control group, PD1 monoclonal antibody group (αPD-1), COG133TFA monotherapy group (αAPOE), PD1 monoclonal antibody and COG133TFA combined group (αAPOE+ αPD-1), 4 mice in each group, and the four groups of mice were treated according to the corresponding groups. Among them, the PD1 monoclonal antibody group: intraperitoneal injection of 6.6mg / kg on the eighth day, and then injected every three days ; COG133TFA single use group: intraperitoneal injection of 1 mg / kg on the second day, and then once every five days; PD1 monoclonal antibody and COG133TFA combined group: intraperitoneal injection of COG133TFA 1 mg / kg on the second day, and then every five days Inject PD1 monoclonal antibody 6.6mg / kg intraperitoneally for eight days, and inject once every three days ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com