Electrolyte food gel and preparation method thereof
A food gel and electrolyte technology, applied in the field of food processing, can solve the problems of aspiration pneumonia, aspiration, and no obvious improvement in diarrhea symptoms, and achieve the effect of reducing aspiration pneumonia and improving diarrhea symptoms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 to 5
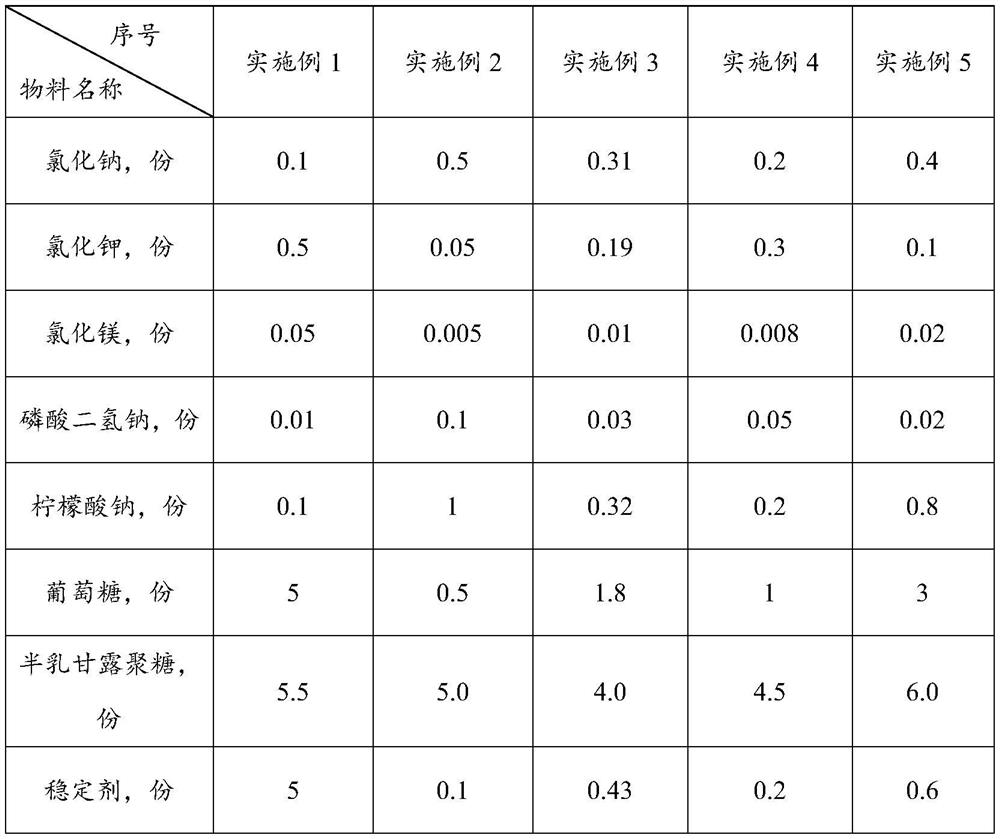
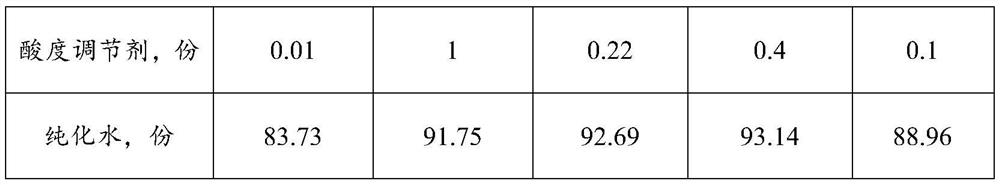
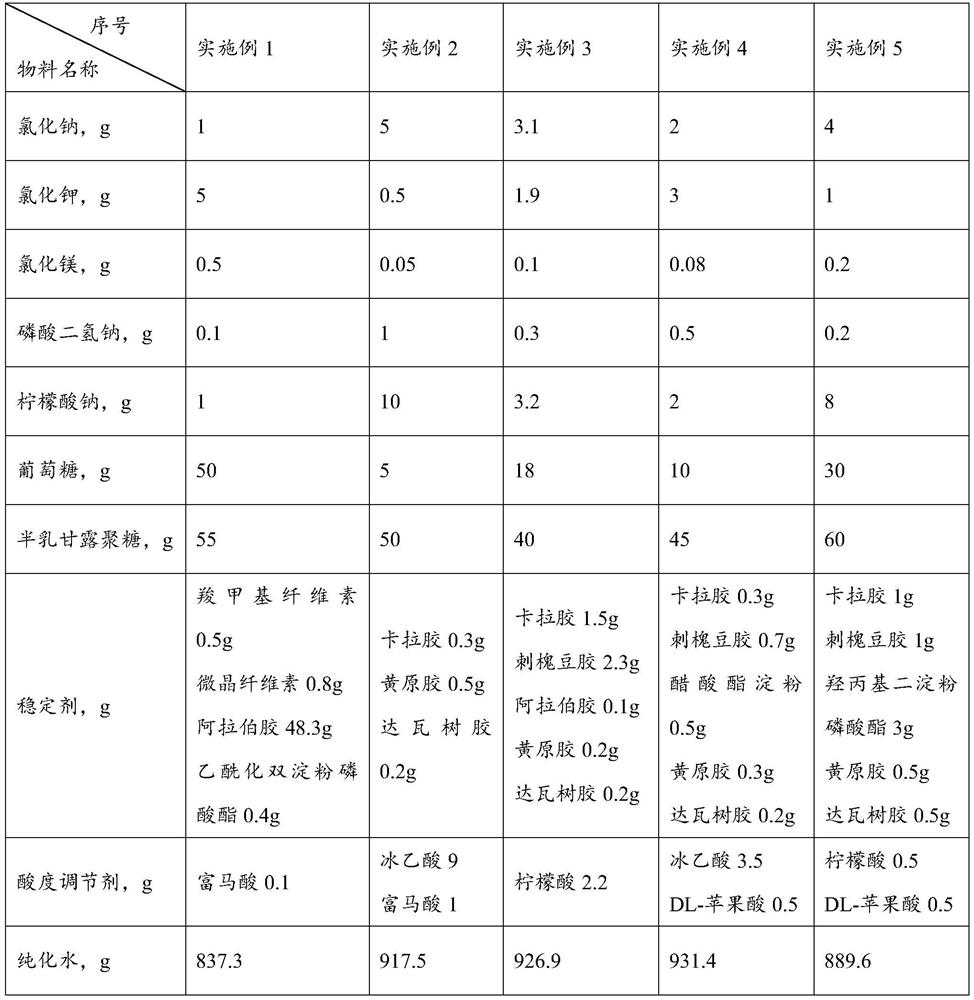
[0047] Embodiment 1-5 provides a kind of electrolyte food gel and preparation method thereof. Wherein, the formula composition of described electrolyte food gel is shown in Table 1-2. The preparation method is as follows: (1) Heating purified water to 75°C, adding a stabilizer, and mixing at 2000rpm for 8 minutes to obtain material I;
[0048] (2) Cool material Ⅰ to 55°C, add glucose, sodium chloride, potassium chloride, magnesium chloride, sodium dihydrogen phosphate, sodium citrate, galactomannan and acidity regulator, and mix at 2000rpm for 12 minutes , get material Ⅱ;
[0049] (3) The material II was subjected to ultra-high temperature instantaneous sterilization, the sterilization temperature was 138°C, and the sterilization time was 9s.
[0050] (4) The ultra-high temperature instant sterilized material II is filled and sealed by the filling machine, and then sterilized for the second time in a water-bath sterilizer. The sterilization temperature is 88 ° C, and the ste...
Embodiment 6
[0057] Prescription: with embodiment 3. The preparation method is as follows:
[0058] (1) Heat the purified water to 70°C, add a stabilizer, and mix at 1000rpm for 5 minutes to obtain material I;
[0059] (2) Cool material Ⅰ to 50°C, add glucose, sodium chloride, potassium chloride, magnesium chloride, sodium dihydrogen phosphate, sodium citrate, galactomannan and acidity regulator, and mix at 1000rpm for 10 minutes , get material Ⅱ;
[0060] (3) The material II is subjected to ultra-high temperature instantaneous sterilization, the sterilization temperature is 130°C, and the sterilization time is 3s.
[0061] (4) After the ultra-high temperature instant sterilized material II is filled and sealed by the filling machine, it is sterilized for the second time in a water-bath sterilizer at a temperature of 85°C and a sterilization time of 20 minutes. .
Embodiment 7
[0063] Prescription: with embodiment 3. The preparation method is as follows:
[0064](1) Heat purified water to 80°C, add stabilizer, and mix at 3000rpm for 10 minutes to obtain material I;
[0065] (2) Cool material Ⅰ to 60°C, add glucose, sodium chloride, potassium chloride, magnesium chloride, sodium dihydrogen phosphate, sodium citrate, galactomannan and acidity regulator, and mix at 3000rpm for 15 minutes , get material Ⅱ;
[0066] (3) The material II is subjected to ultra-high temperature instantaneous sterilization, the sterilization temperature is 150°C, and the sterilization time is 15s.
[0067] (4) After the ultra-high temperature instant sterilized material II is filled and sealed by the filling machine, it is sterilized for the second time in a water-bath sterilizer. The sterilization temperature is 90°C and the sterilization time is 30 minutes. .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com