Application of saponin in preparation of anti-mycoplasma bovis product
A technology of Mycoplasma bovis and saponins, applied in the application field of saponin compounds in the preparation of anti-Mycoplasma bovis products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
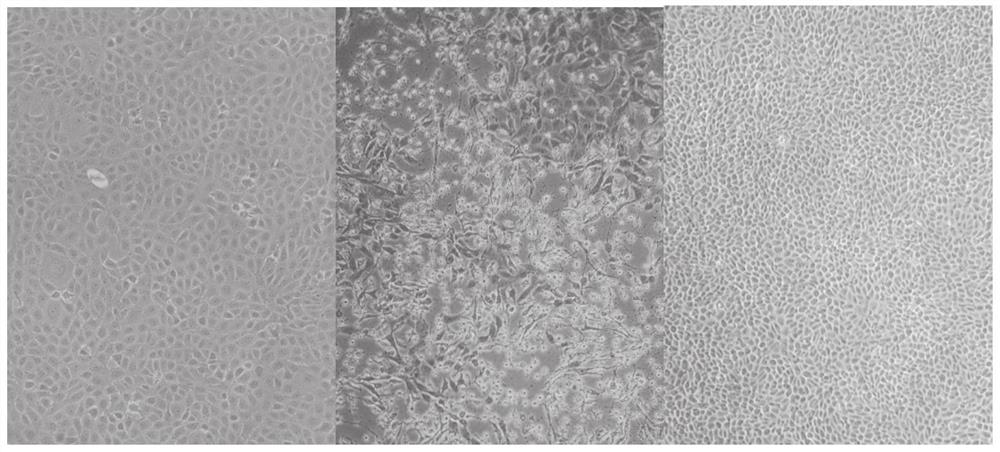
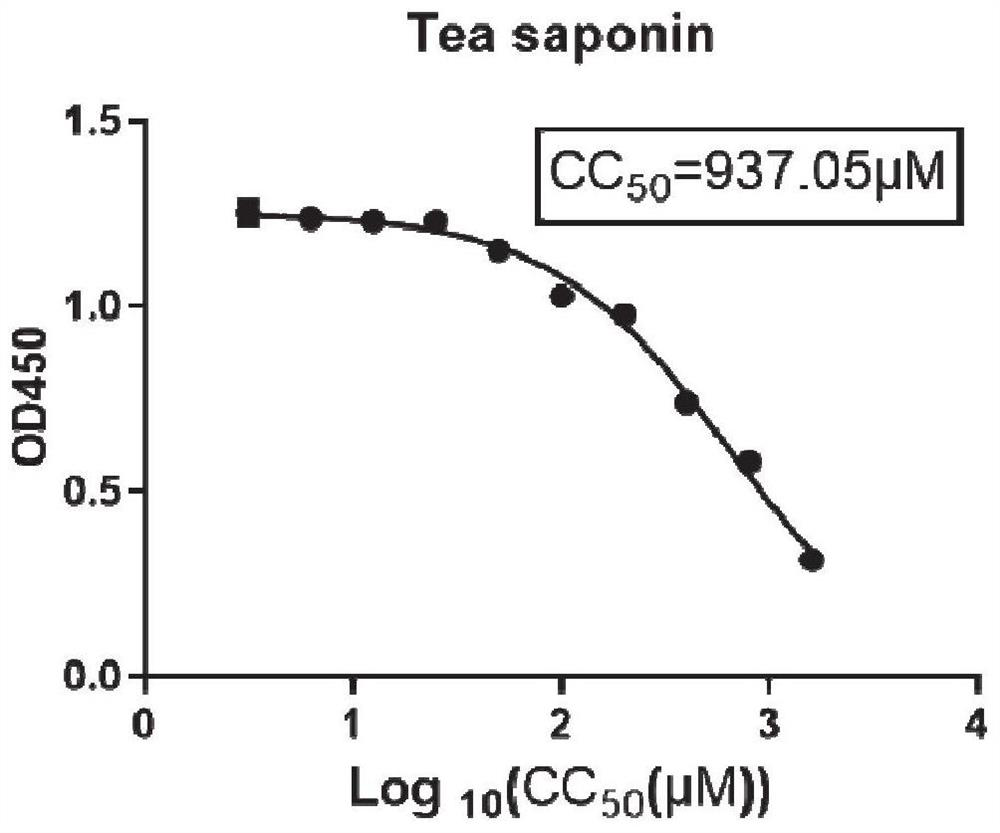
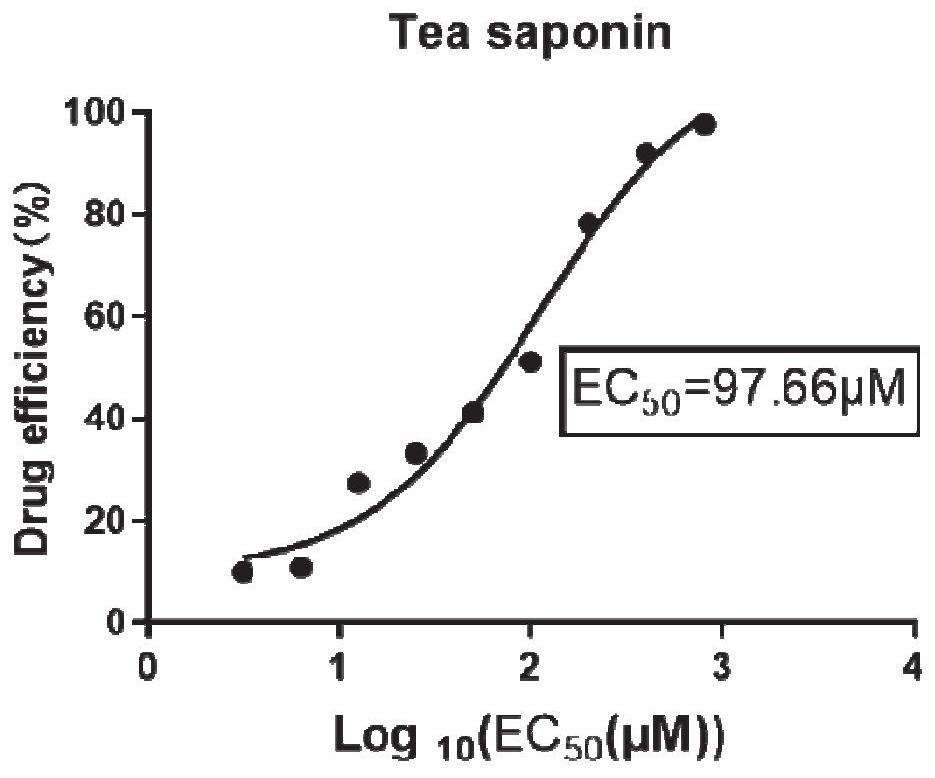
[0067] Embodiment 1 tea saponin suppresses the effect verification of bovis mycoplasma
[0068] 1. Toxicity test of tea saponin on MDBK cells:
[0069] MDBK cells are susceptible cells to M. bovis. Therefore, first detect the cytotoxicity of tea saponin to MDBK cells, the specific experimental steps are as follows:
[0070] (1) Inoculate 100 μL cells (MDBK 5000 cells / well) in a 96-well plate.
[0071] (2) After culturing for about 12 hours, the next step of drug addition analysis was carried out. Discard the medium, add 100 μL of 2% FBS DMEM containing different drug concentrations to each well, and make 3 parallels for each concentration. At the same time, control wells: add 100 μL of 2% FBS DMEM medium containing 0.9% DMSO. Zero well: no cells are plated.
[0072] (3) At 37°C, 5% CO 2 After culturing for 48 h under the condition, the medium in the well was discarded. Wash twice with 100 μL PBS to exclude the influence of drugs on CCK8 response. Add 100 μL DMEM medium...
Embodiment 2 7
[0081]Embodiment 2 Sodium aescinate inhibits Mycoplasma bovis effect verification
[0082] 1. Toxicity test of sodium aescinate on MDBK cells:
[0083] Firstly, the cytotoxicity of sodium aescinate to MDBK cells was detected, and the specific experimental steps were as follows:
[0084] (1) Inoculate 100 μL cells (MDBK 5000 cells / well) in a 96-well plate.
[0085] (2) After culturing for about 12 hours, the next step of drug addition analysis was carried out. Discard the medium, add 100 μL of 2% FBS DMEM containing different drug concentrations to each well, and make 3 parallels for each concentration. At the same time, control wells: add 100 μL of 2% FBS DMEM medium containing 0.9% DMSO. Zero well: no cells are plated.
[0086] (3) At 37°C, 5% CO 2 After culturing for 48 h under the condition, the medium in the well was discarded. Wash twice with 100 μL PBS to exclude the influence of drugs on CCK8 response. Add 100 μL DMEM medium + 10 μL CCK8 solution to each well.
...
Embodiment 3
[0095] Embodiment 3 Diosgenin inhibits Mycoplasma bovis effect verification
[0096] 1. Toxicity test of diosgenin on MDBK cells:
[0097] Firstly, the cytotoxicity of diosgenin to MDBK cells was detected, and the specific experimental steps were as follows:
[0098] (1) Inoculate 100 μL cells (MDBK 5000 cells / well) in a 96-well plate.
[0099] (2) After culturing for about 12 hours, the next step of drug addition analysis was carried out. Discard the medium, add 100 μL of 2% FBS DMEM containing different drug concentrations to each well, and make 3 parallels for each concentration. At the same time, control wells: add 100 μL of 2% FBS DMEM medium containing 0.9% DMSO. Zero well: no cells are plated.
[0100] (3) At 37°C, 5% CO 2 After culturing for 48 h under the condition, the medium in the well was discarded. Wash twice with 100 μL PBS to exclude the influence of drugs on CCK8 response. Add 100 μL DMEM medium + 10 μL CCK8 solution to each well.
[0101] (4) 37°C, 5%...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com