General model for predicting performance of organic compound and prediction method
A technology of organic compounds and general models, applied in the field of computational chemistry, can solve the problems of model uncertainty, poor prediction ability, poor prediction ability, etc., and achieve the effect of high accuracy and large uncertainty.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
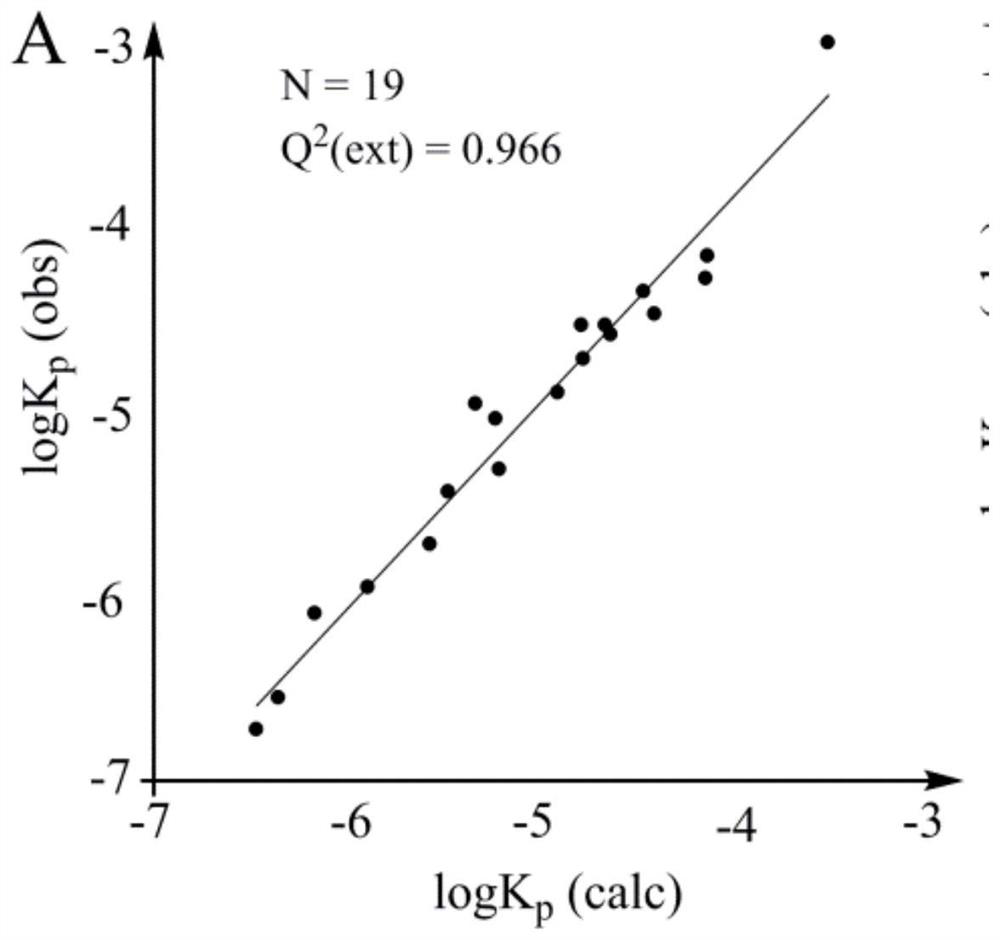
[0062] The present embodiment provides a method for predicting the permeability of human skin by using the LFER model, comprising the following steps:
[0063] (1) Provide 32 known organic compounds, the human skin permeability logK of the known organic compounds p (K p is permeation velocity, unit cm / s) is known, and the chemical structural formula of described known organic compound is known;
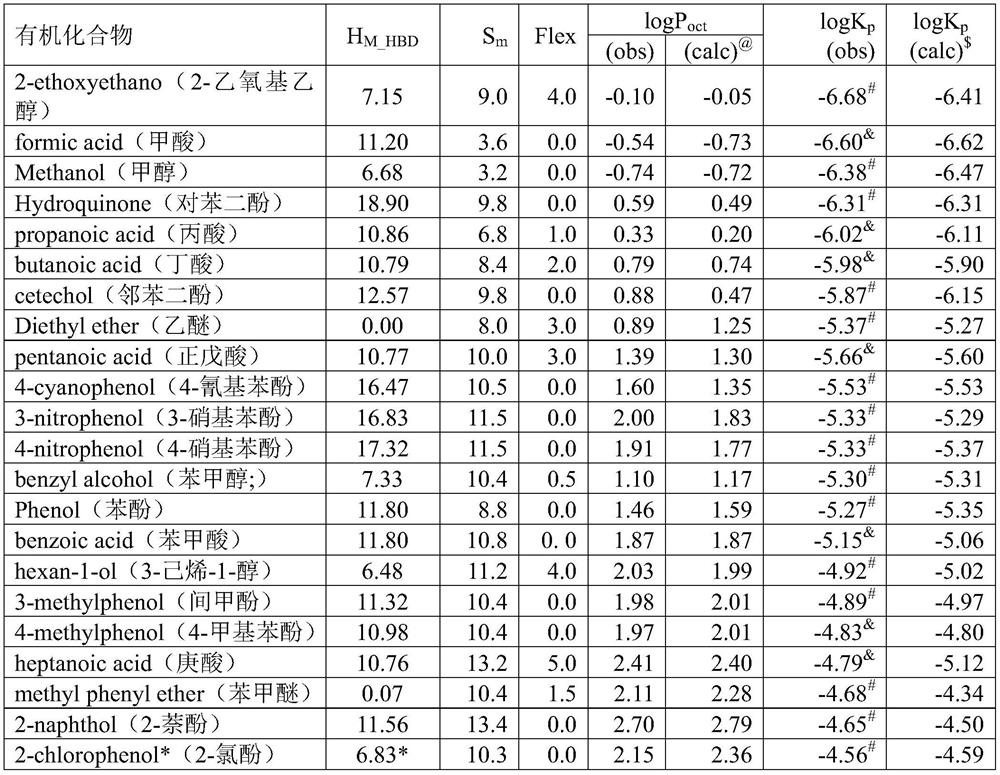
[0064] (2) experimental detection obtains the logP of described known organic compound oct , and obtain the H of the known organic compound according to the structural formula of the known organic compound M_HBD , S m and Flex; as shown in Table 1;
[0065] Table 1
[0066]
[0067]
[0068] $ logK p The calculated value of is calculated by the following model: logK p =0.6157logP oct +0.0156S m -0.0626H M_HBD -0.0988Flex-5.646
[0069] (3) with the logK of the known organic compound p is the dependent variable, logP oct 、H M_HBD , S m and Flex are independent var...
Embodiment 2
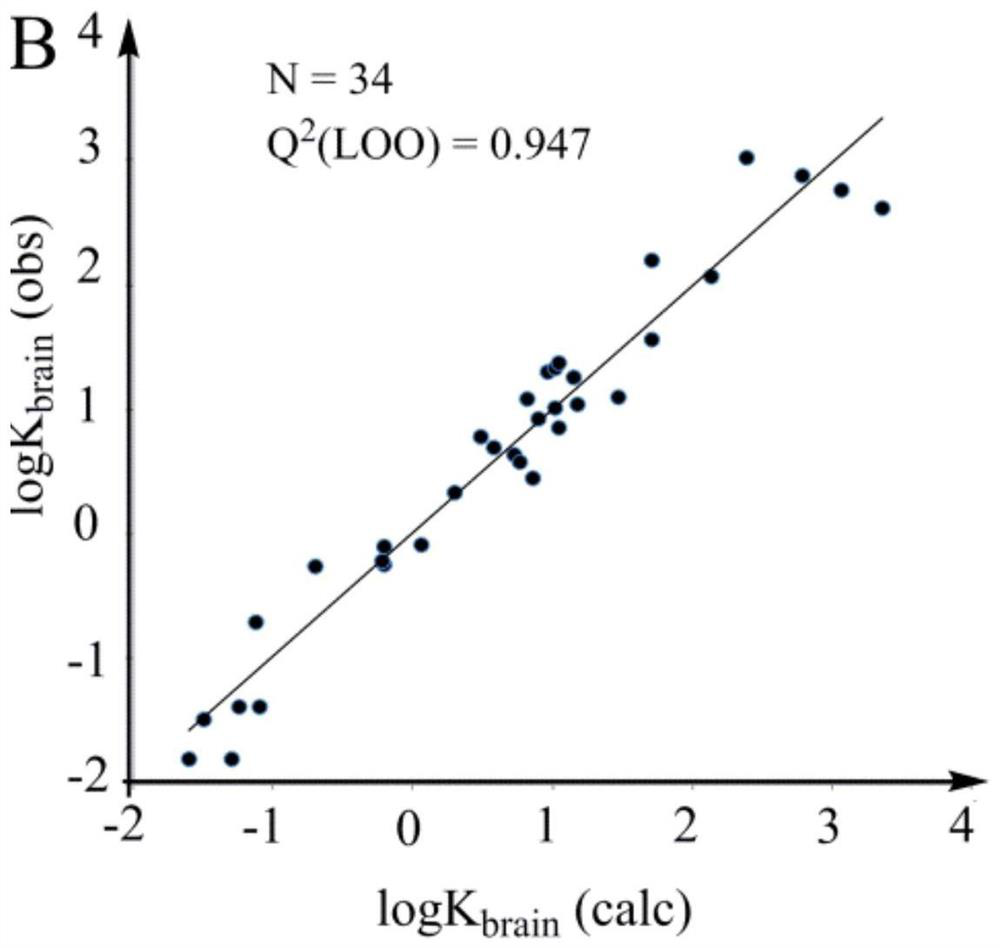
[0080] The present embodiment provides a method for predicting the distribution performance of volatile organic compounds between air and human brain by using the LFER model, comprising the following steps:
[0081] (1) Provide 34 known volatile organic compounds, the logarithm logK of the partition coefficient of said known volatile organic compounds between air and human brain brain Known, the chemical structural formula of the known volatile organic compound is known;
[0082] (2) experimental detection obtains the logP of described volatile known organic compound 16 , and obtain the H of the known volatile organic compound according to the structural formula of the known volatile organic compound M_HBD , S m and Flex;
[0083] (3) with the logK of the known volatile organic compound brain is the dependent variable, logP 16 、H M_HBD , S m and Flex are independent variables, and the constant b in formula Ⅰ is calculated by multiple linear regression method 1 , b 2 ,...
Embodiment 3
[0093] The present embodiment provides a method for predicting the partition coefficient of organic compounds between aniline and water using the LFER model, comprising the following steps:
[0094] (1) provide known organic compound, the logarithm logP of described known organic compound partition coefficient between aniline and water aln Known, the chemical structural formula of the known organic compound is known;
[0095] (2) experimental detection obtains the logP of described known organic compound 16 , and obtain the S of the known organic compound according to the structural formula of the known volatile organic compound m ;
[0096] (3) With the logP of the known organic compound aln is the dependent variable, logP 16 and S m As an independent variable, the constant b in formula Ⅰ is calculated by multiple linear regression method 1 , b 2 ;b 3 , b 4 is zero; the following model is obtained:
[0097] logP aln =0.4695logP 16 +0.1506S m +0.10 (IV);
[0098]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com