A kind of heparin-coated drainage tube for reducing intraocular pressure and preparation method thereof
A heparin coating, drainage tube technology, applied in coatings, catheters, ophthalmic treatment and other directions, can solve the problem of no allergy, inflammation or coagulation, easy to cause allergy, inflammation and coagulation, increase platelet activity factor and other problems, achieve Prevention of scars or blood clots, high safety, and efficacy in reducing complications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0045] The present invention will now be described in detail with reference to the accompanying drawings.
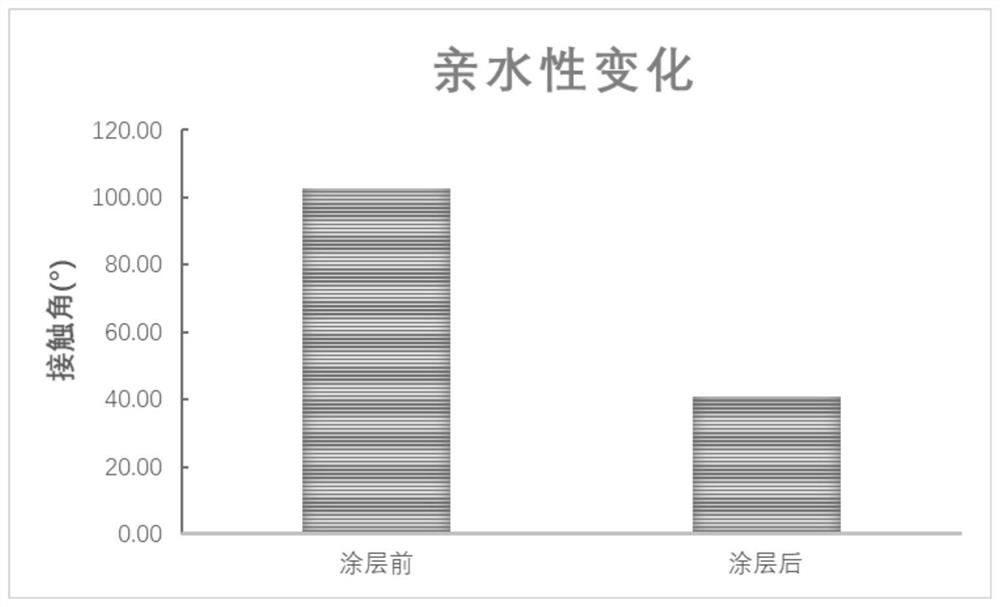
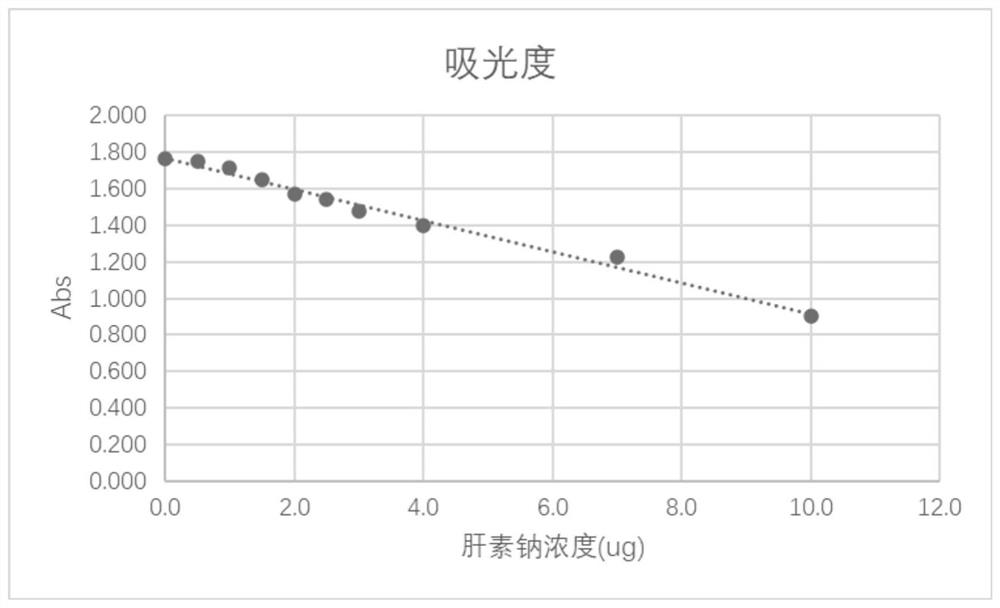
[0046] A heparin-coated drainage tube for reducing intraocular pressure of the present invention includes a base and a heparin coating, the base is made of a biopolymer material, and the heparin coating is covalently attached to the surface of the base through a grafting technique Branch heparin formation. The inner diameter of the drainage tube is 20-150 μm, the outer diameter is 100-500 μm, and the length of the drainage tube is 2-12 mm. Preferably, the inner diameter of the drainage tube is 50-80 μm, the outer diameter is 120-250 μm, and the length of the drainage tube is 3-8 mm.
[0047] A preparation method of a heparin-coated drainage tube for reducing intraocular pressure of the present invention, for preparing the above-mentioned drainage tube, comprises the following steps:
[0048] S1: Preparation of drainage tube base:
[0049] S1.1: Hydrophilic treatment o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com