5-cyano-8-acylaminoquinoline compound and preparation method thereof
A technology for amidoquinolines and compounds, applied in the field of 5-cyano-8-amidoquinolines and their preparation, to avoid metal catalysts and avoid competing reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
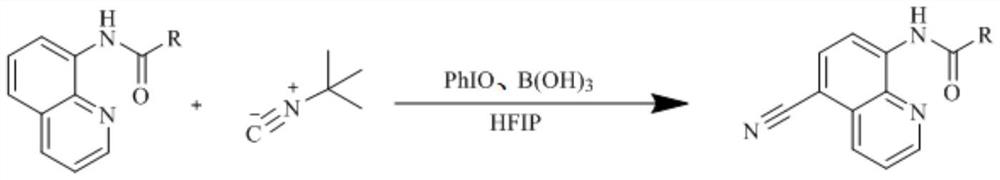
[0018] This specific embodiment provides a kind of preparation method of 5-cyano group-8-amidoquinoline compound, comprises the following steps: in reactor, adds 8-amidoquinoline, tert-butyl isonitrile, iodosobenzene ( Abbreviated as PhIO), boric acid (chemical formula is B(OH) 3 ) and hexafluoroisopropanol (abbreviated as HFIP), then stirred and reacted at 60-70°C for 12-16h to obtain the 5-cyano-8-amidoquinoline compound;
[0019] The obtained 5-cyano-8-amidoquinoline compound is further purified, including: adding water to the prepared 5-cyano-8-amidoquinoline compound, and then adding ethyl acetate to extract 3 -4 times to obtain the organic phase, the organic phase is distilled under reduced pressure to obtain the crude product, and the crude product is separated and purified by column chromatography; the material ratio of the water and the 8-acylaminoquinoline is (75-100 ) mL:1mmol, the volume ratio of the water to the ethyl acetate is (1.5-2):1. The mol ratio of descr...
Embodiment 1
[0026] This embodiment proposes a 5-cyano-8-acetylaminoquinoline, which is prepared by the following steps: add 0.2mmol 8-acetylaminoquinoline, 0.6mmol tert-butylisonitrile, 0.3mmol Iodophenone, 0.6mmol boric acid and 2mL hexafluoroisopropanol, and then add a No. 5 magnet, pass condensed water through the condenser tube from bottom to top, and then stir the reactor at 60°C for 12h to obtain the 5-cyano -8-Acetamidoquinoline;
[0027] The obtained 5-cyano-8-acetylaminoquinoline was further purified, including: adding 15 mL of water to the obtained 5-cyano-8-acetylaminoquinoline, and then adding 10 mL of ethyl acetate to extract 3 times to obtain Organic phase, the organic phase was distilled under reduced pressure to obtain a crude product, and the crude product was separated and purified by column chromatography to obtain purified 5-cyano-8-acetylaminoquinoline with a yield of 50%.
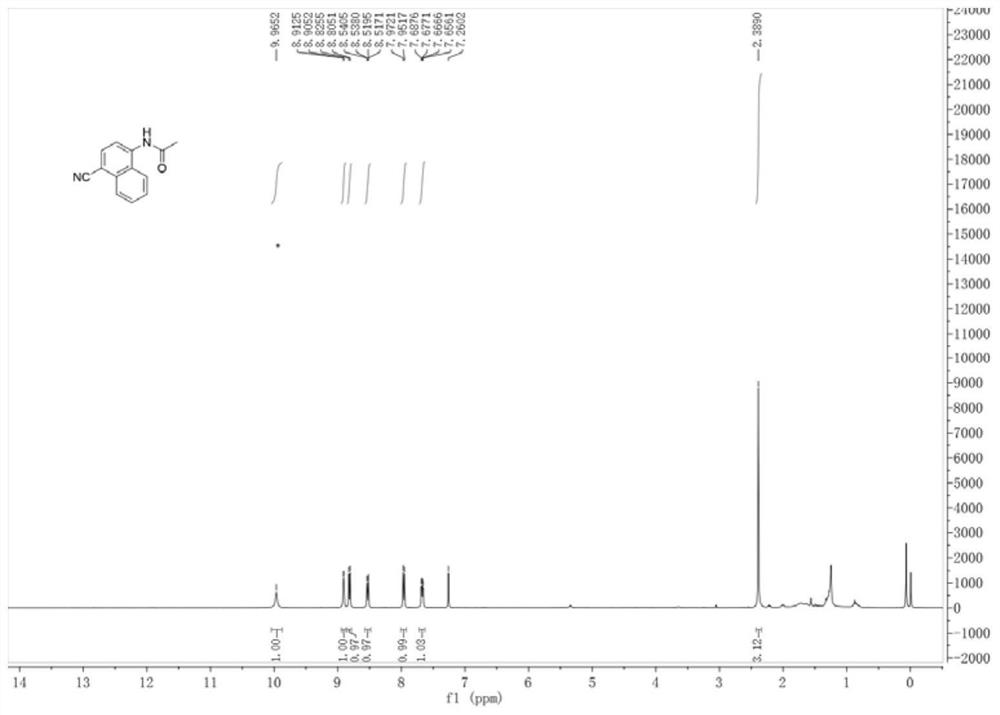
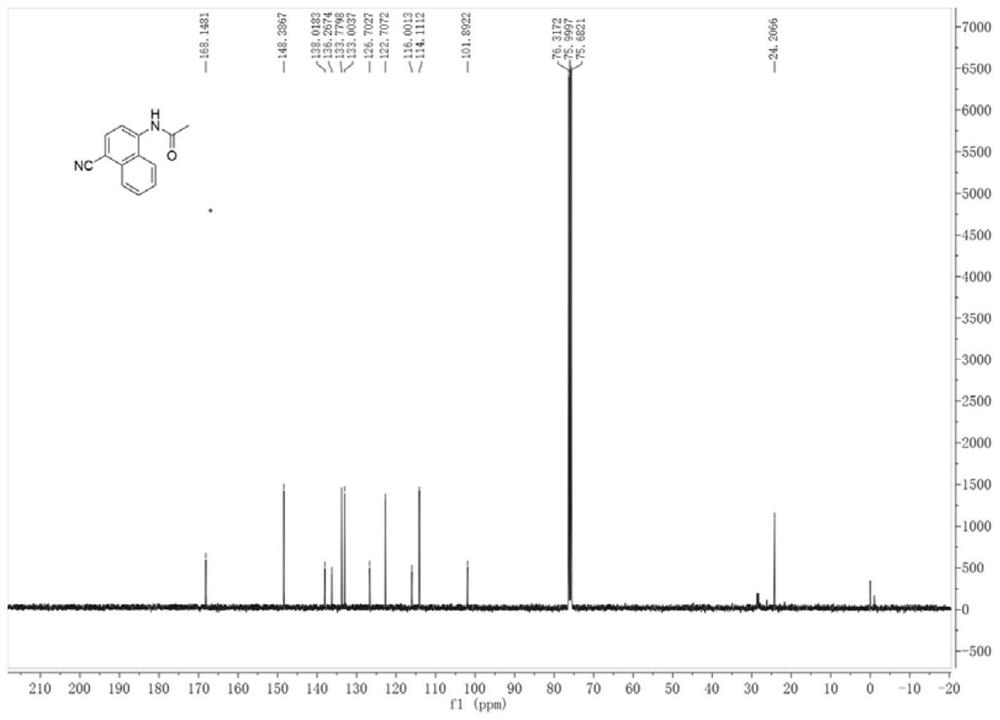
[0028] combine figure 1 and 2 The structure of the product was determined by H NMR and C NM...
Embodiment 2
[0032] This embodiment proposes a 5-cyano-8-acetylaminoquinoline, which is prepared by the following steps: add 0.2mmol 8-acetylaminoquinoline, 0.7mmol tert-butylisonitrile, 0.35mmol Iodosobenzene, 0.7mmol boric acid and 2.5mL hexafluoroisopropanol, and then add a No. 5 magnet, pass the condensed water through the condenser tube from bottom to top, and stir the reactor at 65°C for 14h to obtain the 5- Cyano-8-acetylaminoquinoline;
[0033] The obtained 5-cyano-8-acetylaminoquinoline was further purified, including: adding 15 mL of water to the obtained 5-cyano-8-acetylaminoquinoline, and then adding 10 mL of ethyl acetate to extract 3 times to obtain Organic phase, the organic phase was distilled under reduced pressure to obtain a crude product, and the crude product was separated and purified by column chromatography to obtain purified 5-cyano-8-acetylaminoquinoline with a yield of 48.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com