Application of inosine in preparation of medicines for preventing and treating tuberculosis
A tuberculosis and drug technology, applied to the application field of inosine in the preparation of drugs for preventing and treating tuberculosis, can solve the problems of down-regulation, unreported application, unclear action and mechanism, etc., to promote clearance, inhibit in vivo survival, inhibit Effects of exacerbation of tuberculous granulomas
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] In this example, C57BL / 6 mice were used to infect the H37Rv model to analyze the effect of inosine on tuberculosis. The specific experimental steps and results are as follows:
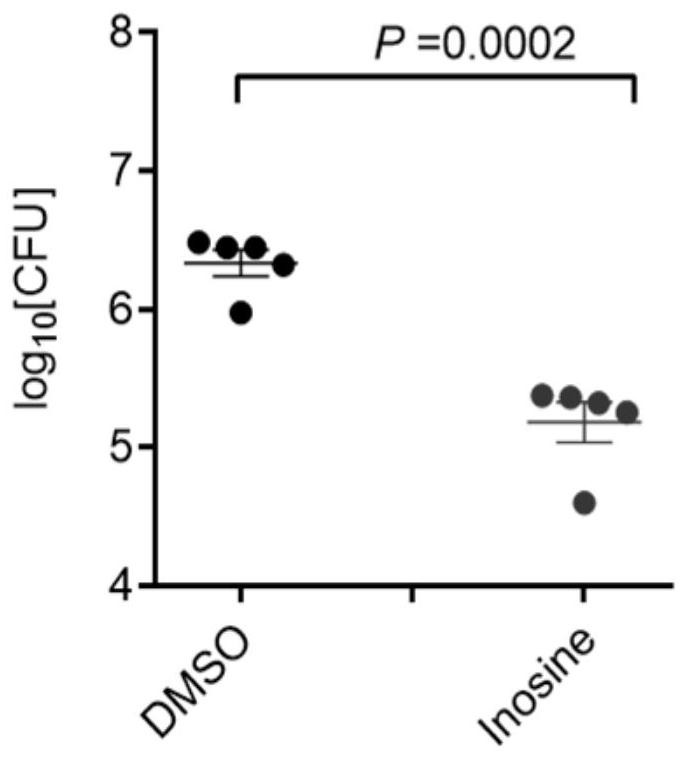
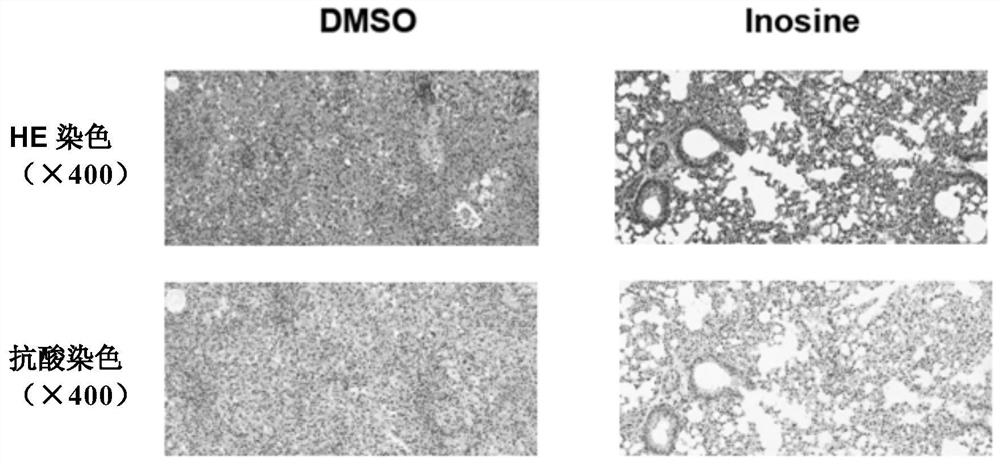
[0033] Six to eight-week-old female C57BL / 6 mice, 6 in each group, were infected with H37Rv strain by intranasal drip, about 200 CFU / mouse. After 3 weeks of infection, the control group added 1% DMSO to the drinking water, and the inosine group added 1% inosine to the drinking water, and the mice were sacrificed after 4 weeks of administration. The effect of inosine on tuberculosis infection in mice was analyzed by detecting the following indicators:
[0034] ① Bacterial load in the lungs: Collect lungs under sterile conditions, add PBS homogenate, inoculate on 7H10 agar medium after 10-fold dilution, and incubate at 37°C for 4 weeks, observe the growth of H37Rv, calculate the bacterial load in the lungs, and find that infection After H37Rv mice drank inosine, the bacterial load in the lungs wa...
Embodiment 2
[0038] In this example, the zebrafish infection model is used to analyze the effect of inosine on mycobacterial diseases, especially granuloma. The specific experimental process and results are as follows:
[0039] Wild-type zebrafish (strain AB) was purchased from the China Zebrafish Resource Center, reared in a recirculation culture system, and transferred to a toxicology system for mycobacterium marinum infection experiments, while ensuring the standard conditions for zebrafish rearing and infection (water temperature about 28 ℃, pH about 7.4, conductivity about 1,500μS). After adult zebrafish were anesthetized with 0.1% tricaine, they were infected with Mycobacterium marinum by intraperitoneal injection. The amount of infected bacteria was about 200 CFU. One week later, the control group was orally administered DMSO, and the inosine group was orally administered 0.01 mg inosine. They were sacrificed 1 week after administration, and the effect of inosine on the development ...
Embodiment 3
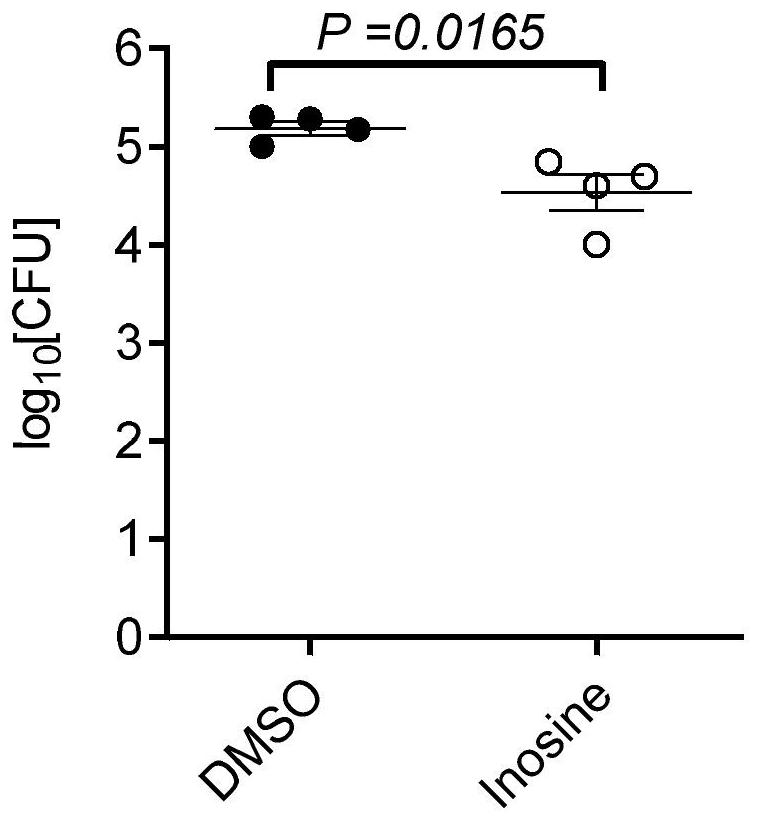
[0044] In this embodiment, the effect of inosine on the survival of intracellular Mtb in macrophages was analyzed using the in vitro infection model of macrophages. The specific experimental process and results are as follows:
[0045] Take the primary peritoneal macrophages of wild-type mice, and culture them with complete 1640 medium (containing 10% FBS + 1% penicillin-streptomycin) at 37°C for 4 hours. Glycosides were used to make the final concentration of 50 μM, and H37Rv was infected 12 hours later, MOI=5. After 3 hours of infection, the supernatant was removed, the cells were washed three times with PBS to remove extracellular bacteria, and the number of bacteria entering the cells was counted by CFU. After another part of the cells were washed, 1640 medium containing DMSO or 50 μM inosine was added to continue culturing for 24 hours, and the survival of intracellular bacteria was detected by CFU. It was found that inosine significantly promoted the intracellular clear...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com