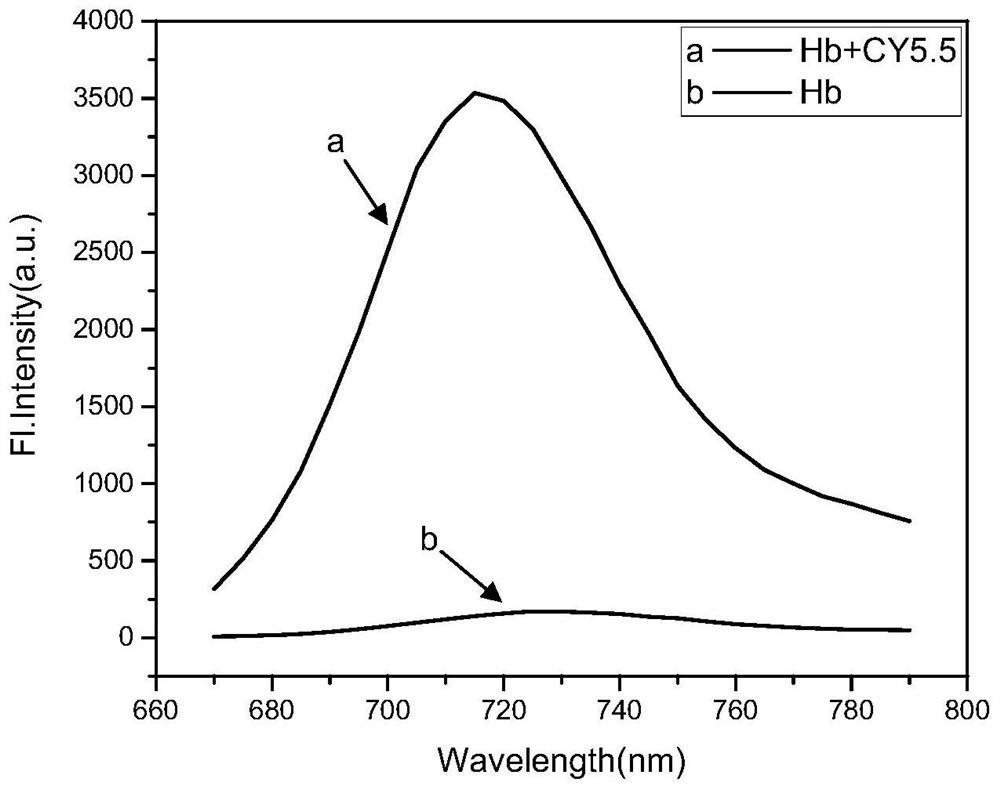
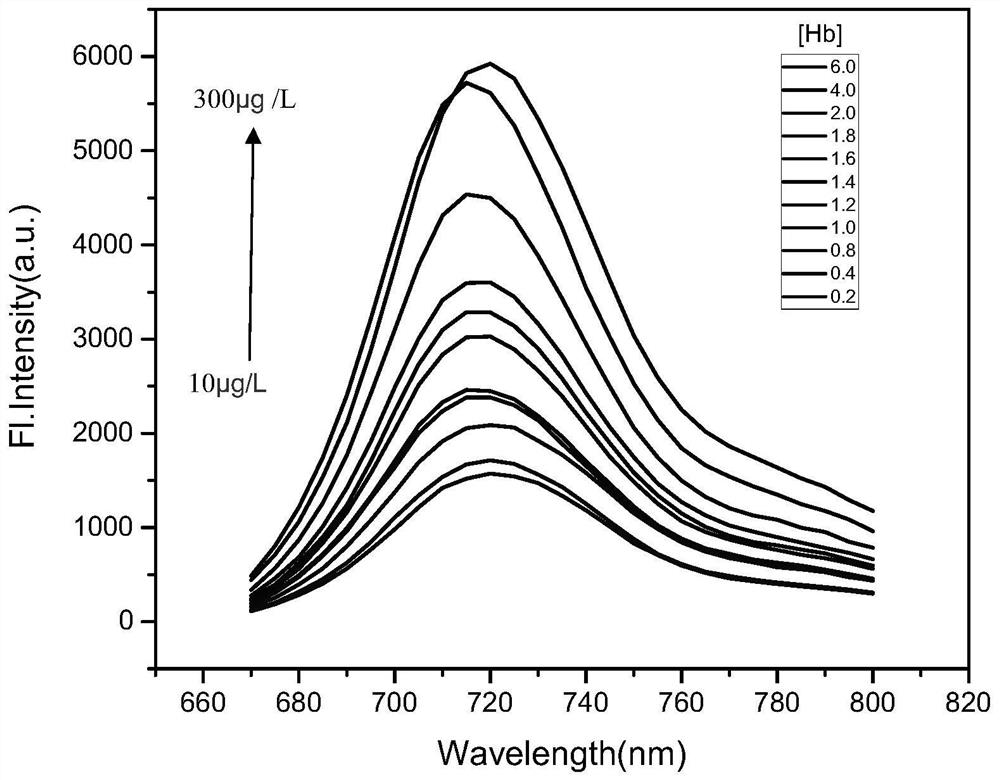
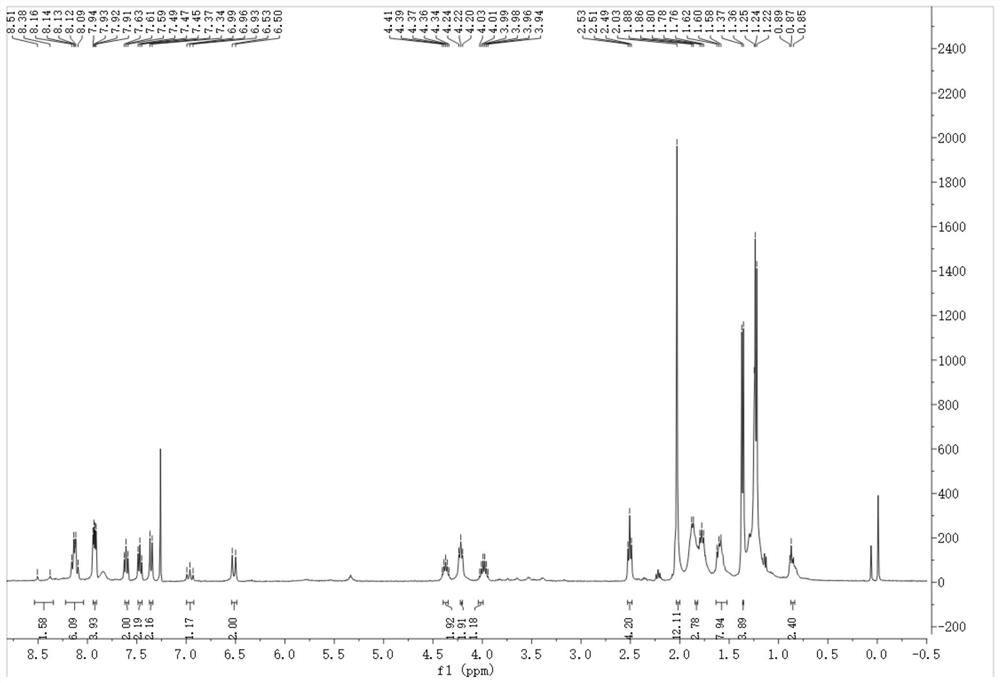
Adenine-based cyanine probe, and preparation method and application thereof
An adenine and probe technology, which is applied in the field of adenine-based cyanine probe and its preparation, can solve the problems of high toxicity, low selectivity and low sensitivity, and achieves high sensitivity, easy control of reaction conditions and simple synthesis method. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] (1) Material 1 (0.5000g, 2.57mmol) and material 2 (0.2214g, 2.57mmol) were refluxed at 120°C for 2.5 hours under acetic acid (8ml) and nitrogen protection conditions. Ethyl ester developer (volume ratio 4:1) was passed through a silica gel column to obtain intermediate 3 (0.5100 g, 2.44 mmol, yield 94.94%).
[0025] (2) Intermediate 3 (0.5100g, 2.44mmol) and raw material 4 (2.2700g, 11.6mmol) were refluxed at 110°C for 36 hours under acetonitrile (8ml) and nitrogen protection conditions, and dissolved with 3ml of dichloromethane after removing the solvent. After adding 3 ml of diethyl ether to precipitate a solid, the solid 5 (0.6610 g, 2.04 mmol, yield 83.61%) was collected by filtration.
[0026] (3) Dissolve Intermediate 5 (0.6610g, 2.04mmol) and Intermediate 6 (0.4986g, 2.24mmol) in a mixed solution of 8mL acetic acid and 8mL acetic anhydride, reflux at 120°C for 4 hours under nitrogen, and remove solvent to obtain the crude solid intermediate 7 (1.0105g, 2.04mmol,...
Embodiment 2
[0034] (1) Material 1 (0.2500g, 1.29mmol) and material 2 (0.1107g, 1.29mmol) were refluxed at 115°C for 2 hours under the conditions of acetic acid (4ml) and nitrogen protection, and the solvent was removed after the reaction, and the mixture was washed with petroleum ether and acetic acid Ethyl ester developer (volume ratio 4:1) was passed through a silica gel column to obtain intermediate 3 (0.2318 g, 1.11 mmol, yield 86.05%).
[0035] (2) Intermediate 3 (0.2318g, 1.11mmol) and raw material 4 (1.0293g, 5.3mmol) were refluxed at 105°C for 30 hours under acetonitrile (4ml) and nitrogen protection, and dissolved in 1.5ml of dichloromethane after removing the solvent Subsequently, 1.5 ml of diethyl ether was added to precipitate a solid, and the solid 5 (0.3305 g, 1.02 mmol, yield 91.89%) was collected by filtration.
[0036] (3) Intermediate 5 (0.3305g, 1.02mmol) and Intermediate 6 (0.2493, 1.12mmol) were dissolved in a mixed solution of 4mL acetic acid and 4mL acetic anhydride...
Embodiment 3
[0040] (1) Material 1 (0.1250g, 0.645mmol) and material 2 (0.0554g, 0.645mmol) were refluxed at 110°C for 2 hours under acetic acid (2ml) and nitrogen protection conditions. Ethyl ester developer (volume ratio 4:1) was passed through a silica gel column to obtain intermediate 3 (0.1172 g, 0.56 mmol, yield 86.82%).
[0041] (2) Intermediate 3 (0.1172g, 0.56mmol) and raw material 4 (0.5071g, 2.6mmol) were refluxed at 100°C for 24 hours under acetonitrile (2ml) and nitrogen protection, and dissolved in 1.0ml of dichloromethane after removing the solvent Subsequently, 1.0 ml of diethyl ether was added to precipitate a solid, and the solid 5 (0.1653 g, 0.51 mmol, yield 91.07%) was collected by filtration.
[0042] (3) Intermediate 5 (0.1653g, 0.51mmol) and Intermediate 6 (0.1247, 0.56mmol) were dissolved in a mixed solution of 2mL acetic acid and 2mL acetic anhydride, and refluxed at 110°C for 3 hours under nitrogen to obtain a crude solid intermediate 7 (0.2527 g, 0.51 mmol, 99.9...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap