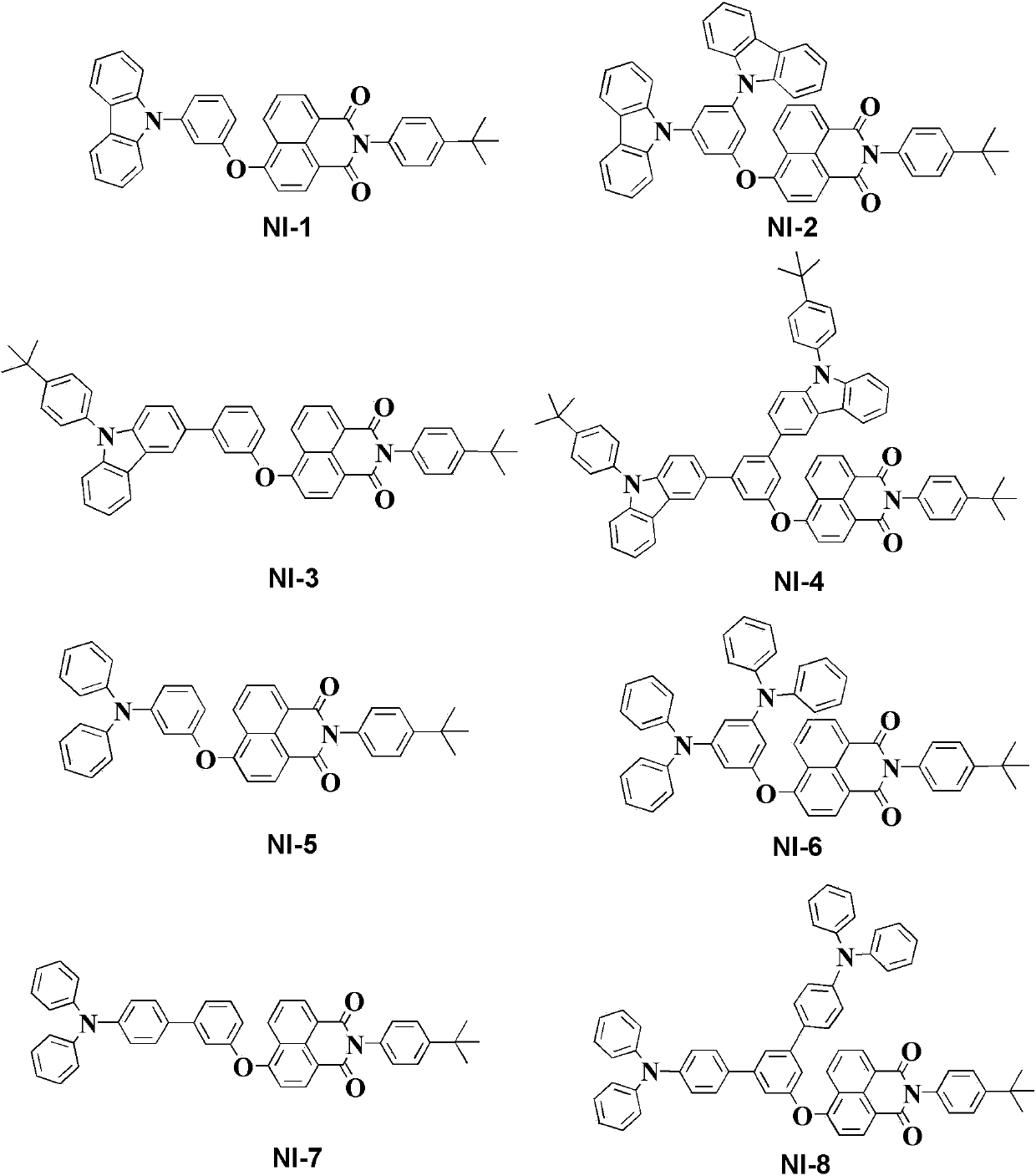
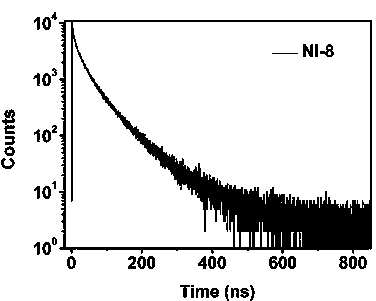
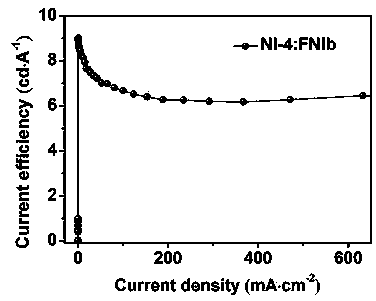
Novel 1,8-naphthalimide derivative compound organic electroluminescent main material and device
A technology of naphthalimide and host material, applied in the field of organic electroluminescence materials, can solve the problems of unsatisfactory electroluminescence performance and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1: the synthesis of NI-1
[0040]
[0041] 1. Compound 4-bromo-N-(4-tert-butylphenyl)-1,8-naphthalimide ( 1 )Synthesis
[0042] Into a 250 mL three-necked flask, add 5.0 g (18.05 mmol) 4-bromo-1,8-naphthalene anhydride, 3.23 g (21.64 mmol) 4-tert-butylaniline and 150 mL EtOH, heat to reflux for 14 h, and cool to At room temperature, spin off the solvent to obtain a solid, and recrystallize from acetone to obtain white needle-like crystals, with a yield of 81.1%. Melting point: 292-293°C. 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 8.71 (dd, J = 7.2, 0.8 Hz, 1H), 8.65 (dd, J = 8.4, 0.8 Hz, 1H), 8.47 (d, J = 8.0 Hz, 1H), 8.09 (d, J = 7.6 Hz, 1H), 7.91 (t, J 1 = 7.2 Hz, J 2 = 8.4 Hz, 1H), 7.57 (d, J = 8.8 Hz, 2H), 7.24 (d, J = 8.8 Hz, 2H), 1.38 (s, 9H).
[0043] 2. 4-(3-bromophenoxy)-N-(4-tert-butylphenyl)-1,8-naphthalimide ( 2 )Synthesis
[0044] To a 250 mL three-necked bottle, add 0.5 g (1.23 mmol) 1 , 0.30 g (1.9 mmol) m-bromophenol, 0...
Embodiment 2
[0047] Embodiment 2: the synthesis of NI-2
[0048]
[0049] 1. 4-(3,5-dibromophenoxy)-N-(4-tert-butylphenyl)-1,8-naphthalimide ( 3 )Synthesis
[0050] To a 250 mL three-necked bottle, add 0.5 g (1.23 mmol) 1 , 0.30 g (1.9 mmol) m-bromophenol, 0.29 g (2.1 mmol) K 2 CO 3 React with 30 mL of DMF in an oil bath at 100 °C for 4 h, then cool to room temperature, pour the reaction solution into 150 mL of water, and filter with suction to obtain a yellow solid. The product was subjected to silica gel column chromatography (eluent: ethyl acetate / petroleum ether = 1:4) to obtain a yellow solid with a yield of 65 % and a melting point of 274-276 °C. 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 8.77 (d, J = 7.6 Hz, 1H), 8.67 (d, J = 7.2 Hz, 1H), 8.23 (d, J = 7.6 Hz, 2H), 8.08 (d, J = 7.6 Hz, 1H), 7.94 (d, J = 8.0 Hz, 1H), 7.59 (d, J = 8.4 Hz, 1H), 7.45 (t, d, J 1 = 8.8 Hz, J 2 = 7.2 Hz, 1H), 7,6 (d, J = 8.4 Hz, 2H), 7.3 (d, J = 8.4 Hz, 2H), 7.06 (d, J = 7.6 Hz,...
Embodiment 3
[0053] Embodiment 3: the synthesis of NI-3
[0054]
[0055] NI-3 Synthesis
[0056] To a 250 mL round bottom flask, add 0.5 g (0.87 mmol) 2 , 0.81 g (1.91 mmol) 3-(4,4,5,5-tetramethyl-[1,3,4]dioxaborolane-9-(N-(4-tert-butylphenyl ))-9 H -carbazole, 0.03 g (0.026 mmol) Pd (pph 3 ) 4 , 12 mL NaCO 3 (2 M, aq.) and 40 mL of toluene were reacted in an oil bath at 100 °C for 24 h. After the reaction was finished, cool to room temperature, pour the reaction solution into 150 mL of water, and extract with dichloromethane, anhydrous Na 2 SO 4 After drying, spin off the solvent, silica gel column chromatography (eluent: ethyl acetate / petroleum ether = 1 / 10) gave a yellow solid, yield: 57.4%, melting point: 301-302 °C. 1 H NMR (400 MHz, CDCl 3 ) δ (ppm): 8.84-8.81 (dd, J = 9.6, 7.2 Hz, 1H), 8.73-8.71 (dd, J = 8.0, 6.4 Hz, 1H), 8.54-8.52 (d, J = 8.0 Hz, 1H), 8.36 (s, 1H), 8.18-8.16 (d, J = 7.6 Hz, 1H), 7.86-7.82 (t, J = 7.6 Hz,1H), 7.69-7.40 (m,14H), 7.31-7.27 (...
PUM
| Property | Measurement | Unit |
|---|---|---|
| external quantum efficiency | aaaaa | aaaaa |
| external quantum efficiency | aaaaa | aaaaa |
| external quantum efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com