Preparation method and application of ratio type fluorescent probe for peroxynitrite
A technology of peroxynitroso and fluorescent probes, applied in the field of fluorescent probes, can solve the problems of reducing probe sensitivity, interference, short analysis wavelength, etc., and achieve the effect of good spectral response performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Synthesis of fluorescent probes
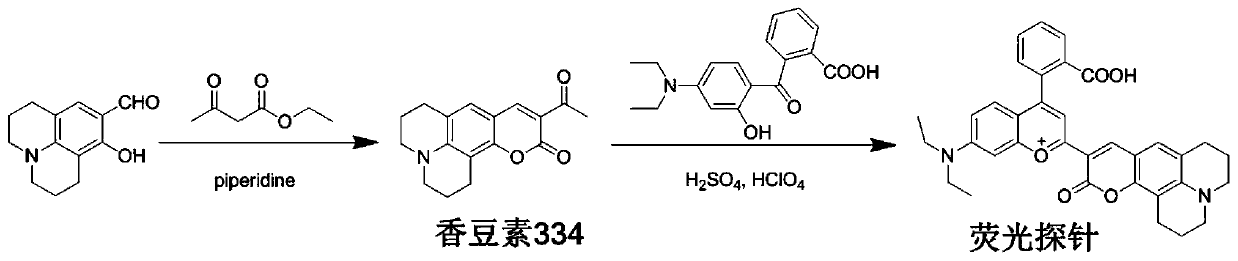
[0027] Synthetic route such as figure 1 . Synthesis of Coumarin 334: In a 100 mL round bottom flask, 8-hydroxyjulolidine-9-carbaldehyde (1.09 g, 5 mmol), ethyl acetoacetate (0.63 mL, 5 mmol) and piperidine (0.035 mL, 0.5mmol) was dissolved in 20.0mL dichloromethane, stirred and refluxed for 6h, stopped the reaction, and the solvent was removed by distillation under reduced pressure, and the thick product was subjected to column chromatography with dichloromethane eluent to obtain a dark yellow solid compound (0.85g, The yield is 60%), which is coumarin 334.
[0028] ONOO - Synthesis of fluorescent probes: In a 100 mL round bottom flask, coumarin 334 (0.42 g, 1.48 mmol) and 4-diethylamino ketoacid (0.46 g, 1.48 mmol) were dissolved in 8 mL of concentrated sulfuric acid, and the reaction mixture Stir at 90°C for 12h to stop the reaction, then cool the reaction mixture to room temperature, pour it into a beaker filled with 50g of ice w...
Embodiment 2
[0030] Fluorescent Probes and ONOO - Solution preparation
[0031] Preparation of probe solution: Weigh a certain amount of probe and dissolve it in methanol to make 1×10 -3 M probe solution. ONOO - Solution preparation: 0.70M H 2 o 2 solution, 0.60M HCl solution, 0.60M NaNO 2 The solution was mixed, and 1.5M NaOH solution was added rapidly, the excess H 2 o 2 Remove with manganese dioxide and store in a freezer at -20°C. Thaw before use, ONOO - The determination of concentration needs to measure the absorbance A of the solution at 302nm, and the calculation formula is: C ONOO -=A / 1.67 (mM).
Embodiment 3
[0033] Fluorescent Probes and ONOO - Determination of the fluorescence spectrum of the action
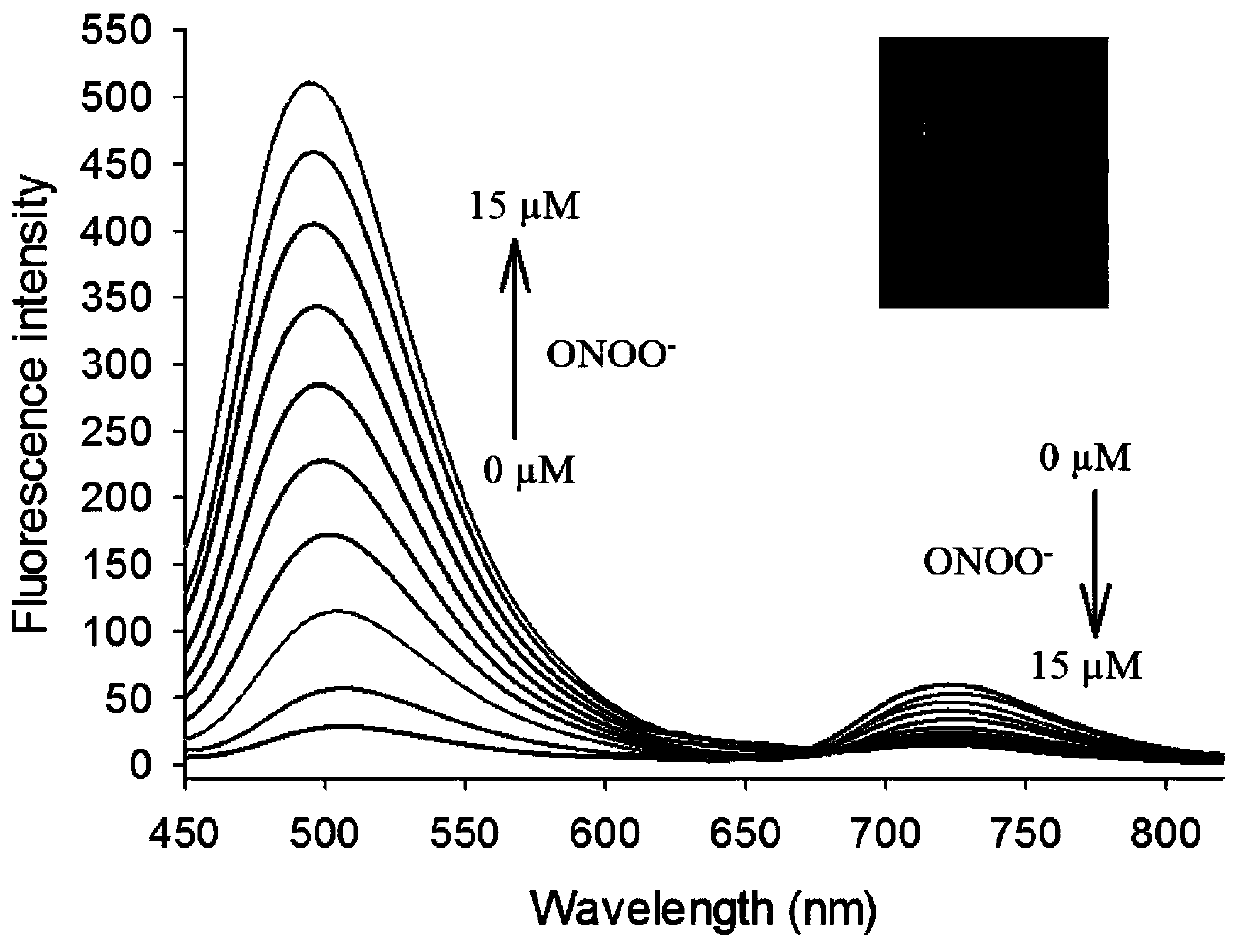
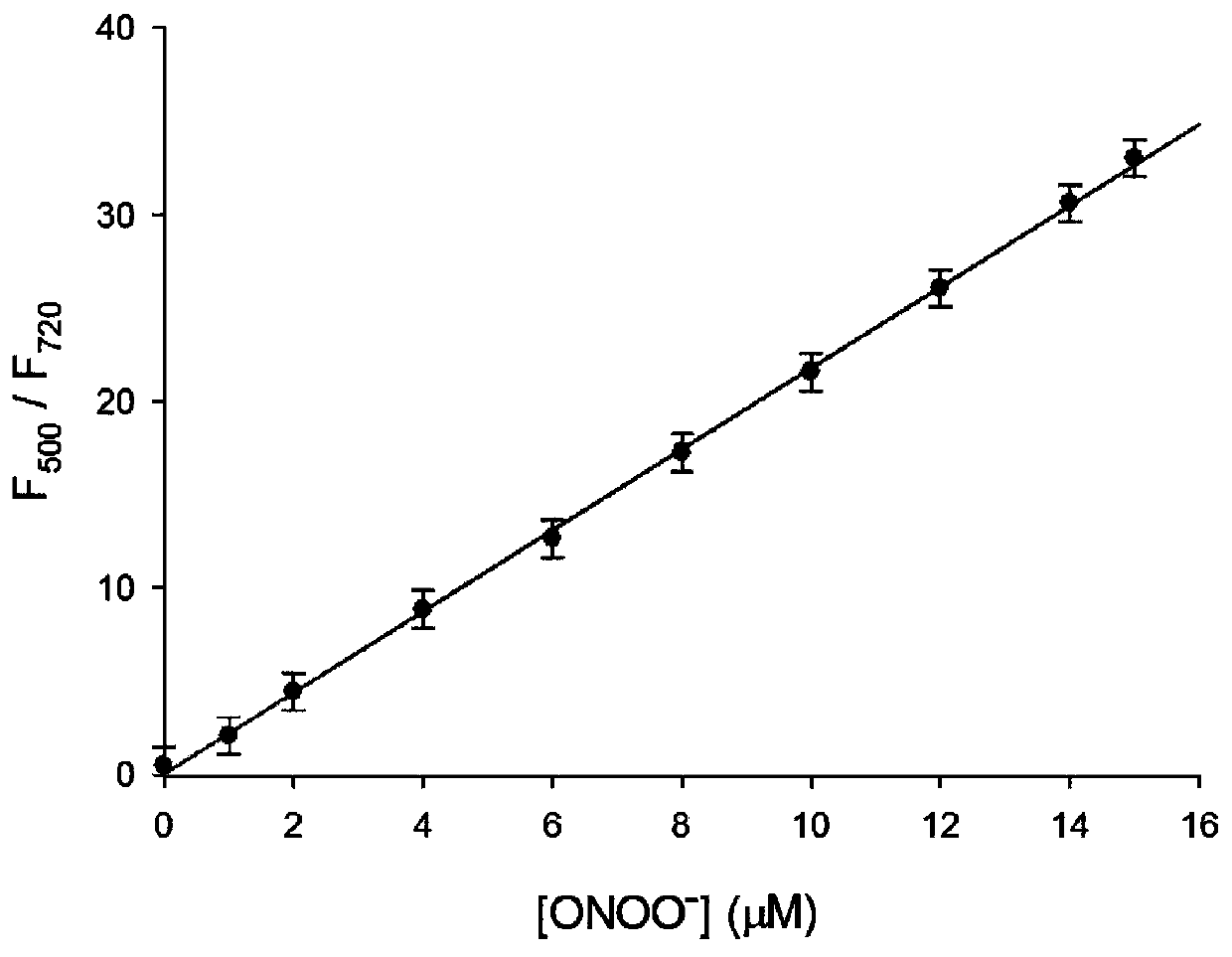
[0034] figure 2 for fluorescent probes with ONOO - The fluorescence spectrum of the action, the concentration of the fluorescent probe is 5 μM, ONOO - The concentrations are 0, 1.0, 2.0, 4.0, 6.0, 8.0, 10, 12, 14, 15 μM in turn. The excitation wavelength is fixed at 440nm, and the emission wavelength range is 450-820nm. The slit width is 5.0 nm / 5.0 nm, and the fluorescence measurement instrument used is a Hitachi F4600 fluorescence spectrophotometer. from figure 2 It can be seen that joining ONOO - Before, fluorescent probes had near-infrared (720nm) fluorescence emission peaks; adding ONOO - After that, a green emission peak appeared in the visible region (500nm). This is because the probe molecules are ONOO - Oxidation, resulting in cleavage, the smaller the conjugated structure, resulting in short-wavelength green fluorescence. And with ONOO - With the increase of th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com