Preparation and application of an open fluorescent probe for detecting carbon monoxide
A fluorescent probe, carbon monoxide technology, applied in the field of fluorescent probes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Synthesis of Fluorescent Probes
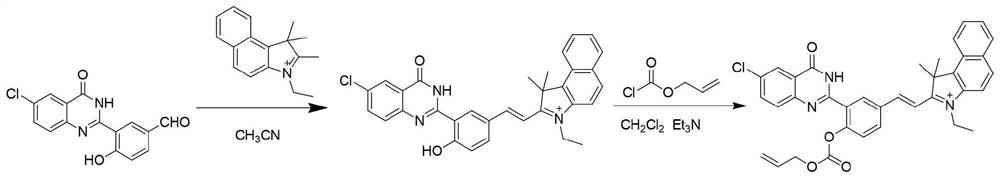
[0027] Synthetic routes such as figure 1 . Synthesis of compound Ct-OH: In a 100 mL round bottom flask, 3-(6-chloro-4-oxo-3,4-dihydroquinazolin-2-yl)-4-hydroxybenzaldehyde (HPQ- CHO) (0.30 g, 1.0 mmol) and 3-ethyl-1,1,2-trimethyl-1H-benzo[e]indole-3-onium (0.35 g, 1.0 mmol) were dissolved in 10 mL of toluene , under the conditions of room temperature and nitrogen protection, firstly add 0.5mL piperidine with a 1mL syringe, then add 0.5mL acetic acid with a 1mL syringe, the reaction mixture was stirred overnight to stop the reaction, and then the reaction solution was mixed with saturated saline and CH 2 Cl 2 Extraction, take the lower organic phase, dry with anhydrous sodium sulfate, filter, and spin dry, the crude product can be CH with a volume ratio of 100:1 to 100:4. 2 Cl 2 / CH 3 OH eluent was used for column chromatography to obtain a green solid compound (0.32 g, yield 50%), which is compound Ct-OH.
[0028] Synthesis of C...
Embodiment 2
[0030] Fluorescent probe and CO solution preparation
[0031] Preparation of probe solution: Weigh a certain amount of probe and dissolve it in dimethyl sulfoxide to make 1×10 -4 M's probe solution. PdCl 2 Preparation of solution: weigh a certain amount of PdCl at the same time 2 Dissolve in double distilled water to make 1×10 -4 M stock solution. Preparation of CO solution: Dissolve a certain amount of CORM-3 in double distilled water, transfer it to a 500mL volumetric flask, add water to the mark, and obtain a concentration of 1.0×10 -3 mol·L -1 CORM-3. will be 1.0 x 10 -3 mol·L -1 The CORM-3 solution was gradually diluted to obtain 1.0 x 10 -4 -1.0×10 -5 mol·L -1 of CORM-3 in water. Combine 1.0 mL of probe stock solution, 1.0 mL of PdCl 2 The stock solution and 1.0 mL of CORM-3 aqueous solution were added to different 10 mL volumetric flasks, and the volume was adjusted with buffer solution to obtain a concentration of 1.0 × 10 -5 mol·L -1 Fluorescent probe s...
Embodiment 3
[0033] Determination of Fluorescence Spectra of Fluorescent Probe Interaction with CO
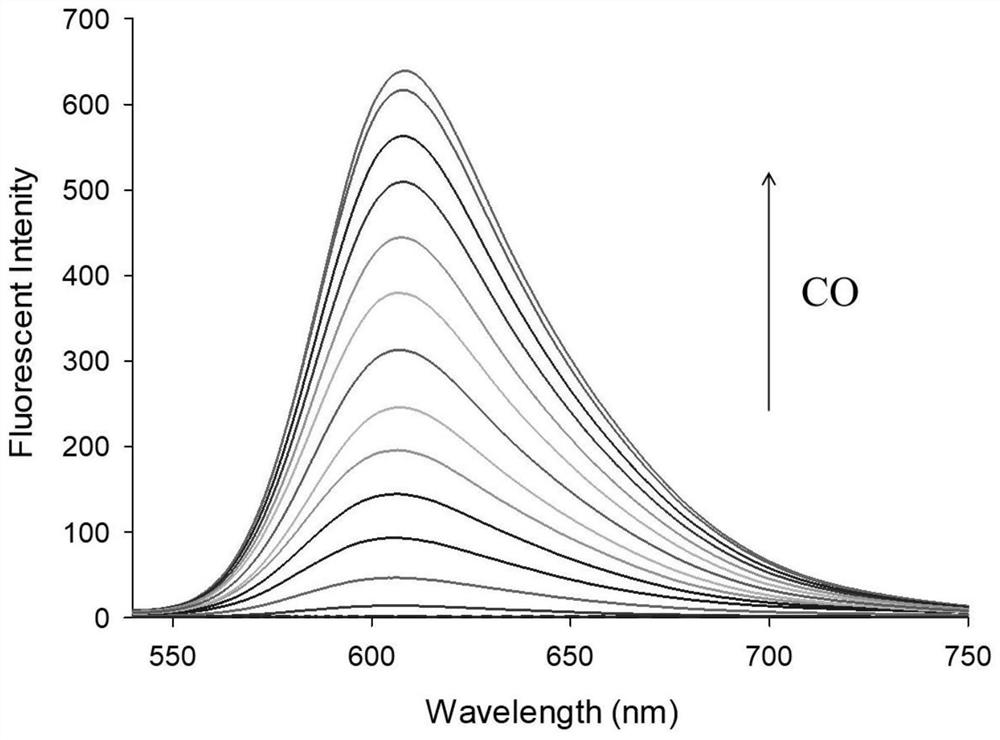
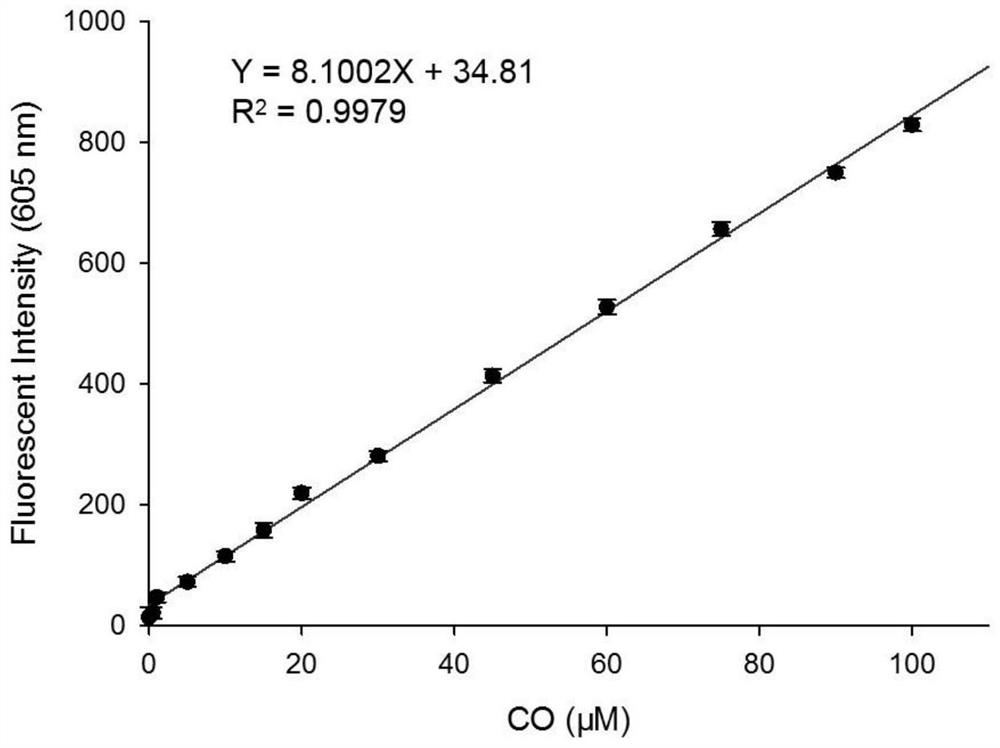
[0034] figure 2 Fluorescence spectra for the interaction of fluorescent probes with CO, fluorescent probes and Pd 2+ The concentrations of CO were all 10 μM, and the CO concentrations were: 0, 1.0, 5.0, 10, 15, 20, 25, 30, 40, 50, 60, 80, 90, 100 μM, respectively. The excitation wavelength was fixed at 570 nm, and the fluorescence emission wavelength ranged from 540 to 750 nm. The slit width is 5.0 nm / 5.0 nm, and the fluorescence measuring instrument used is Hitachi F4600 fluorescence spectrophotometer. from figure 2 It can be seen that due to the quenching effect of allyl chloroformate on the hydroxyl group in the novel fluorophore Ct-OH, it can be found that when the fluorescent probe is added to Pd 2+ After that, there is no obvious emission peak at 605 nm; while adding Pd 2+ After CO and CO, a distinct emission peak appeared at 605 nm. This is because Pd 2+ First reduced by CO ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com