Lnterleukin-2 receptor (IL2R) and interleukin-2 (IL2) variants for specific activation of immune effector cells
A technology of interleukin and IL2, applied in the direction of blood/immune system cells, animal cells, albumin peptides, etc., can solve problems such as limiting the efficacy of cytokines and the influence of resident immune cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0495] Example 1: Construct Design
[0496] To design an interactive pair of human IL2 (hIL2) and interleukin 2 receptor subunit alpha (hIL2RA, CD25), various amino acid substitutions were made at the hIL2:hIL2RA binding interface. In more detail, three pairs of basic and acidic amino acid residues were identified as part of the ionic interactions driving hIL2:hIL2RA binding. The respective residues are swapped in a reciprocal fashion, mutating basic amino acids to acidic amino acids and vice versa. For both hIL2 and the corresponding hIL2RA, up to three amino acid positions were mutated, resulting in four hIL2 variants with predicted altered hIL2RA binding and four hIL2RA mutants with predicted altered hIL2 binding.
[0497] Four different hIL2 variants contain the following amino acid substitutions:
[0498] -hIL2_A3: K35E, K43E and E61K
[0499] -hIL2_A4: K43E and E61K
[0500] -hIL2_A5:E61K
[0501] -hIL2_A8:K43E
[0502] Four different hIL2RA mutants contained the ...
Embodiment 2
[0510] Example 2: mRNA Production
[0511]Cytokines encoding mRNA for in vitro transcription are based on the pST1-T7-AGA-dEarI-hAg-MCS-FI-A30LA70 plasmid-backbone and derived DNA-constructs. These plasmid constructs contain a 5'UTR (untranslated region, a derivative of the 5'-UTR of the Homosapiens hemoglobin subunit α1 (hAg)), a 3'FI element (where F is the amino-terminal enhancer of the split mRNA I is a 136 nucleotide long 3'-UTR segment of the 12S RNA, and I is a 142 nucleotide long segment of the mitochondrial encoded 12S RNA, both identified in Homo sapiens; WO 2017 / 060314) and 100 nucleotides Acid poly(A) tail with a linker after 70 nucleotides.
[0512] Cytokine and serum albumin (hAlb) coding sequences were derived from Homo sapiens and no changes were introduced in the resulting amino acid sequences except for the expected mutations in the hIL2 variants described above (hIL2: NP_000577.2; NCBI Protein Resource [AM1] ). For the cytokine constructs, the hIL2 varian...
Embodiment 3
[0520] Example 3: In vitro expression of RNA-encoded hAlb-hIL2 variants
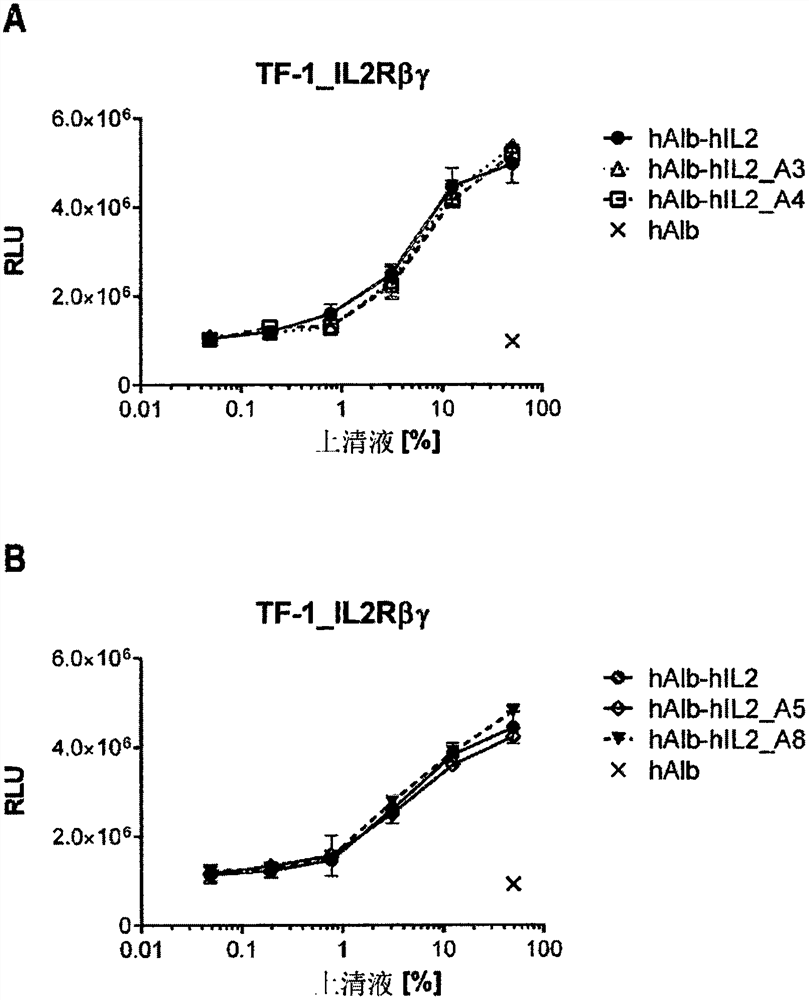
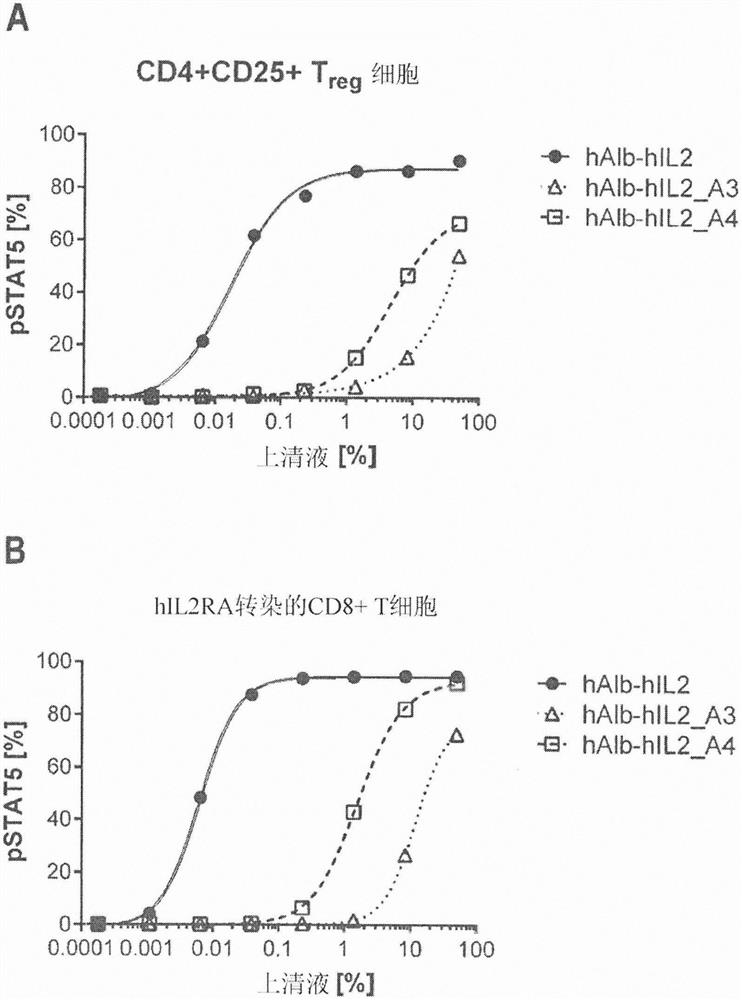
[0521] The resulting hAlb-hIL2-encoding variants were analyzed by transfecting mRNA liposomes into HEK293T / 17 cells and subsequently analyzing CD25-independent activation of reporter cells expressing IL2Rβγ by supernatants containing hAlb-hIL2 variants. In vitro expression of body mRNA ( figure 1 A, B). One day before liposome transfection, 1.2×10 6 HEK293T / 17 cells were seeded in 3 mL of DMEM (Life Technologies GmbH, Cat. No. 31966-021 ) + 10% Fetal Bovine Serum (FBS, Biochrom GmbH, Cat. No. S0115 ) in 6-well plates. For liposome transfection, under sterile and RNase-free conditions, 3 μg of IVT-mRNA was prepared using 400 ng mRNA / μL Lipofectamine MessengerMax (Thermo Fisher Scientific, cat. 2 The dish should be applied to approximately 80% confluent HEK293T / 17 cells. After 20 hours of expression, supernatants were collected under sterile conditions and stored at -20°C until further use. The CD25-i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com