A kind of ortho-sulfonylated arylamine compound and synthetic method thereof
A technology of sulfonylated aromatic amine and synthesis method, applied in the field of organic chemical synthesis, can solve the problems of incomplete conversion of sulfoxide, long reaction time, excessive oxidant, etc., and achieves high synthesis difficulty, good reaction repeatability, and common substrate. good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
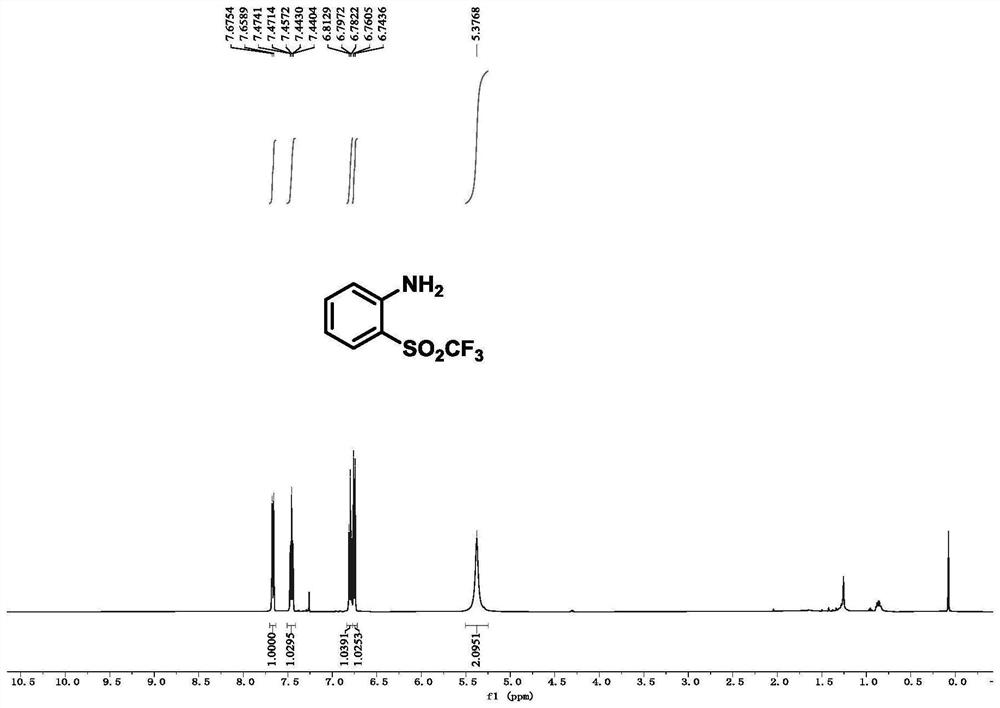
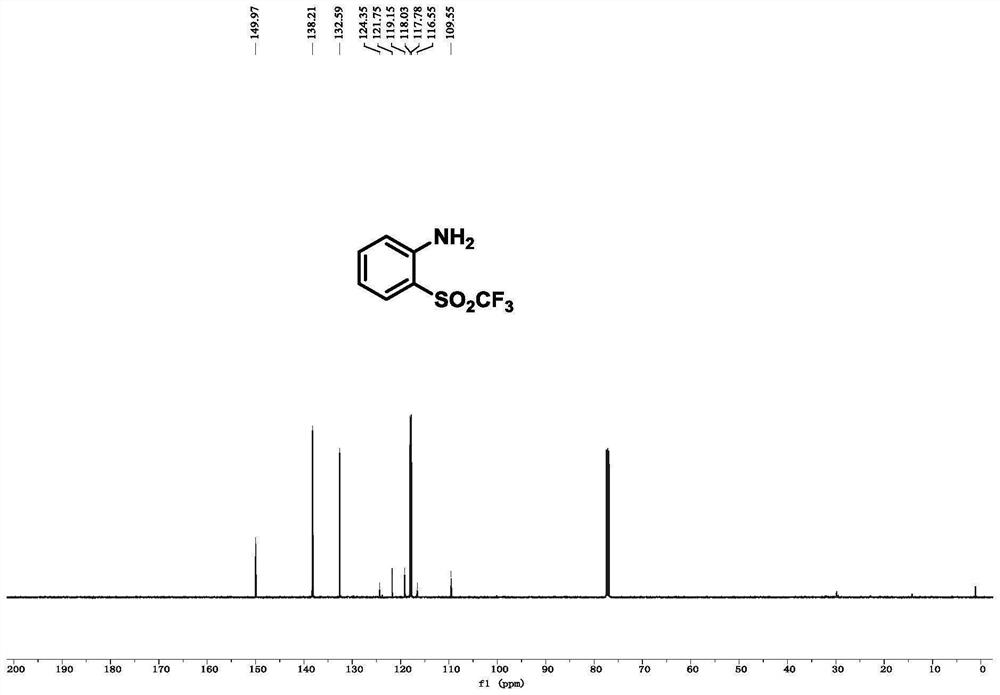
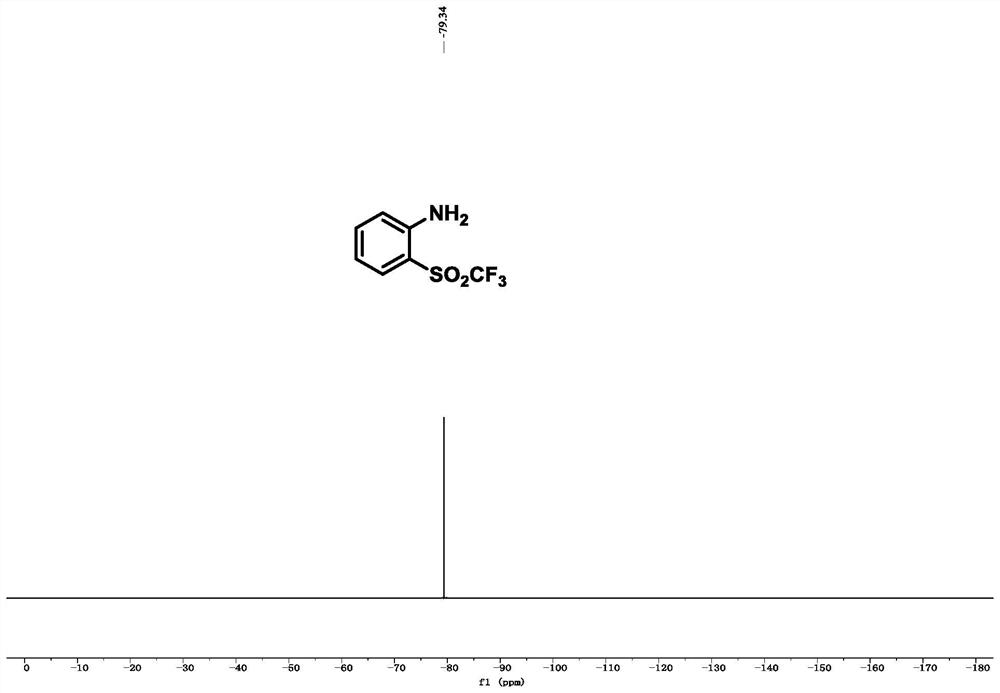
[0078] Example 1, 2-((trifluoromethyl)sulfonyl)aniline
[0079]
[0080] Under nitrogen atmosphere, N-phenylhydroxylamine (0.2 mmol, 1.0 eq.) was dissolved in an ultra-dry solution of dichloromethane (2 mL) and cooled to 0 °C. Then triethylamine (0.3 mmol, 1.5 eq.) was added slowly, and trifluoromethylsulfinyl chloride (0.24 mmol, 1.2 eq.) was added dropwise with stirring. The reaction mixture was stirred at 0°C for 10 minutes. After the reaction was completed, the reaction mixture was diluted with DCM and the solvent was removed by rotary evaporation. The crude product was subjected to column chromatography (eluent: petroleum ether:ethyl acetate=10:1) After purification, pure 2-((trifluoromethyl)sulfonyl)aniline was obtained as a colorless oil with a yield of 87%.
[0081] 1 H NMR (500MHz, CDCl 3 ): δ7.67(d, J=8.2Hz, 1H), 7.51-7.40(m, 1H), 6.93-6.59(m, 2H), 5.38(s, 2H).
[0082] 13 C NMR (126MHz, CDCl 3 ):δ150.0,138.2,132.6,120.5(q,J C-F = 326.6Hz), 118.0, 117.8, 10...
Embodiment 2
[0084] Example 2, 4-chloro-2-((trifluoromethyl)sulfonyl)aniline
[0085]
[0086] Under nitrogen atmosphere, N-(4-chlorophenyl)hydroxylamine phenylhydroxylamine (0.2 mmol, 1.0 eq.) was dissolved in an ultra-dry solution of dichloromethane (2 mL) and cooled to 0 °C. Then triethylamine (0.3 mmol, 1.5 eq.) was added slowly, and trifluoromethylsulfinyl chloride (0.24 mmol, 1.2 eq.) was added dropwise with stirring. The reaction mixture was stirred at 0°C for 10 minutes. After the reaction was completed, the reaction mixture was diluted with DCM and the solvent was removed by rotary evaporation. The crude product was subjected to column chromatography (eluent: petroleum ether:ethyl acetate=10:1) After purification, pure yellow solid 4-chloro-2-((trifluoromethyl)sulfonyl)aniline was obtained with a yield of 92%.
[0087] 1 H NMR (500MHz, CDCl 3 ): δ7.65 (d, J=2.3Hz, 1H), 7.40 (dd, J=8.9, 2.4Hz, 1H), 6.72 (d, J=8.9Hz, 1H), 5.39 (s, 2H).
[0088] 13 C NMR (126MHz, CDCl 3 ): δ...
Embodiment 3
[0090] Example 3, 4-(2-methyl-1,3-dioxolan-2-yl)-2-((trifluoromethyl)sulfonyl)aniline
[0091]
[0092]Under nitrogen atmosphere, N-(4-(2-methyl-1,3-dioxolan-2-yl)phenyl)hydroxylamine (0.2 mmol, 1.0 eq.) was dissolved in ultra-dry dichloromethane ( 2 mL) solution and cooled to 0 °C. Then triethylamine (0.3 mmol, 1.5 eq.) was added slowly, and trifluoromethylsulfinyl chloride (0.24 mmol, 1.2 eq.) was added dropwise with stirring. The reaction mixture was stirred at 0°C for 10 minutes. After the reaction was completed, the reaction mixture was diluted with DCM and the solvent was removed by rotary evaporation. The crude product was subjected to column chromatography (eluent: petroleum ether:ethyl acetate=10:1) Purification to obtain pure 4-(2-methyl-1,3-dioxolan-2-yl)-2-((trifluoromethyl)sulfonyl)aniline as a white solid with a yield of 97%.
[0093] 1 H NMR (500MHz, CDCl 3 ): δ7.78(s, 1H), 7.55(dd, J=8.6, 1.6Hz, 1H), 6.74(d, J=8.6Hz, 1H), 5.40(s, 2H), 4.02(t, J= 6.9Hz, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com