Marker, kit and device for predicting sensitivity of colorectal cancer patients to immunotherapy
An immunotherapy, colorectal cancer technology, applied in the field of cancer, can solve the problems of immunotherapy efficacy deficiency, long working cycle, high technical platform requirements, and achieve the effect of expanding the potential benefit population and reducing the cost of testing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0089] Download the data of 85 patients with advanced colon adenocarcinoma (COAD) who received immune checkpoint inhibitor therapy from the MSKCC data, which includes gene mutation data, treatment regimen data and overall survival (OS) data, as shown in Table 1 Show.
[0090]
[0091]
[0092]
[0093]
[0094]
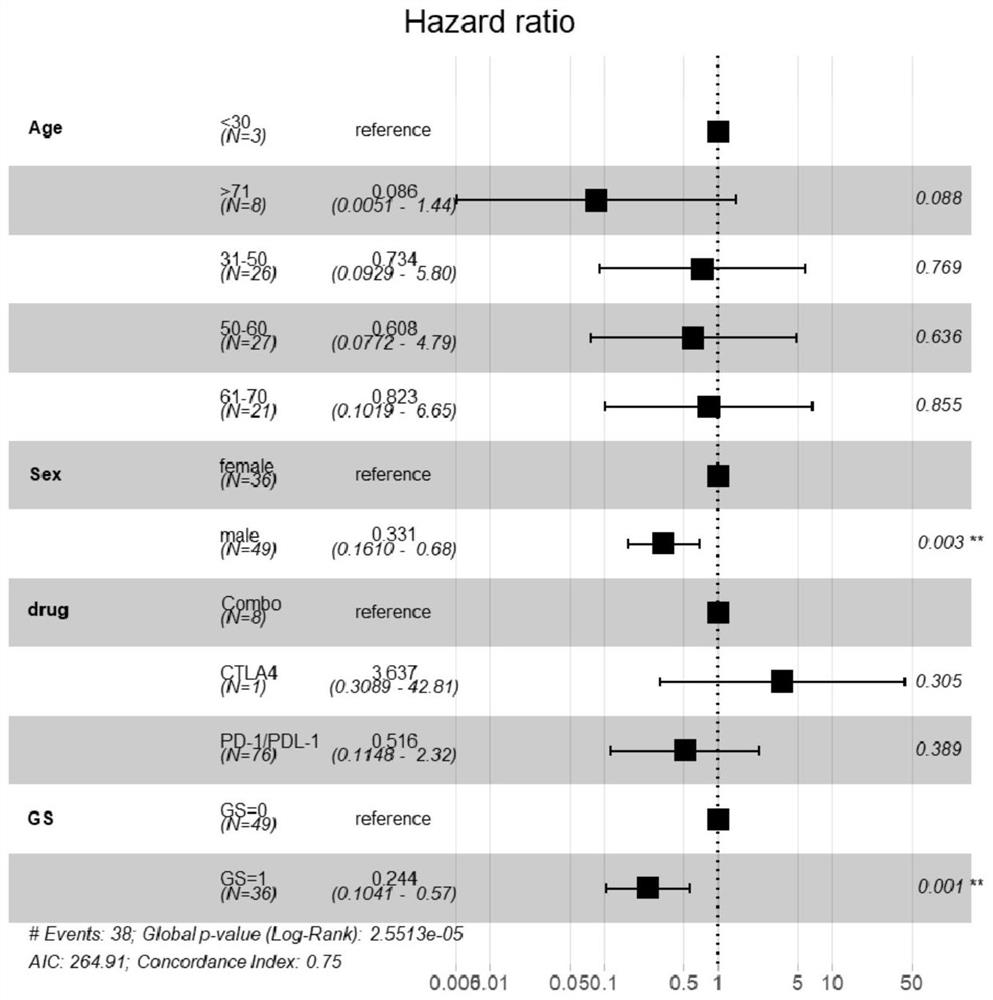
[0095] First, univariate COX analysis was performed on 85 patients with advanced colon adenocarcinoma (COAD) receiving immune checkpoint inhibitor therapy, and it was found that the genes most correlated with overall survival (OS) of immunotherapy (P<0.1) were shared among 85 patients. 6, the specific information is shown in Table 2.
[0096] Table 2
[0097]
[0098] These 6 genes were then divided into two categories: among them, APC and VHL were preliminarily judged as negatively correlated factors for predicting overall survival because the HR value was greater than 1 (but there was only one case of VHL mutant type, so it was difficult to judge ...
Embodiment 2
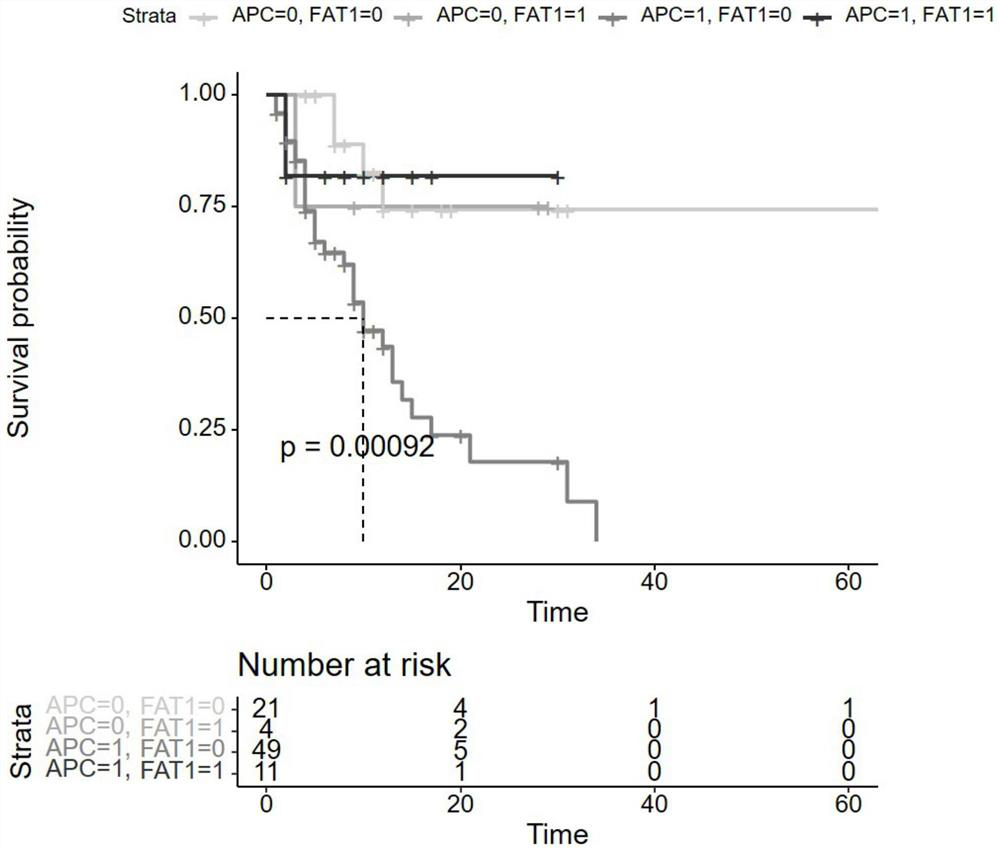
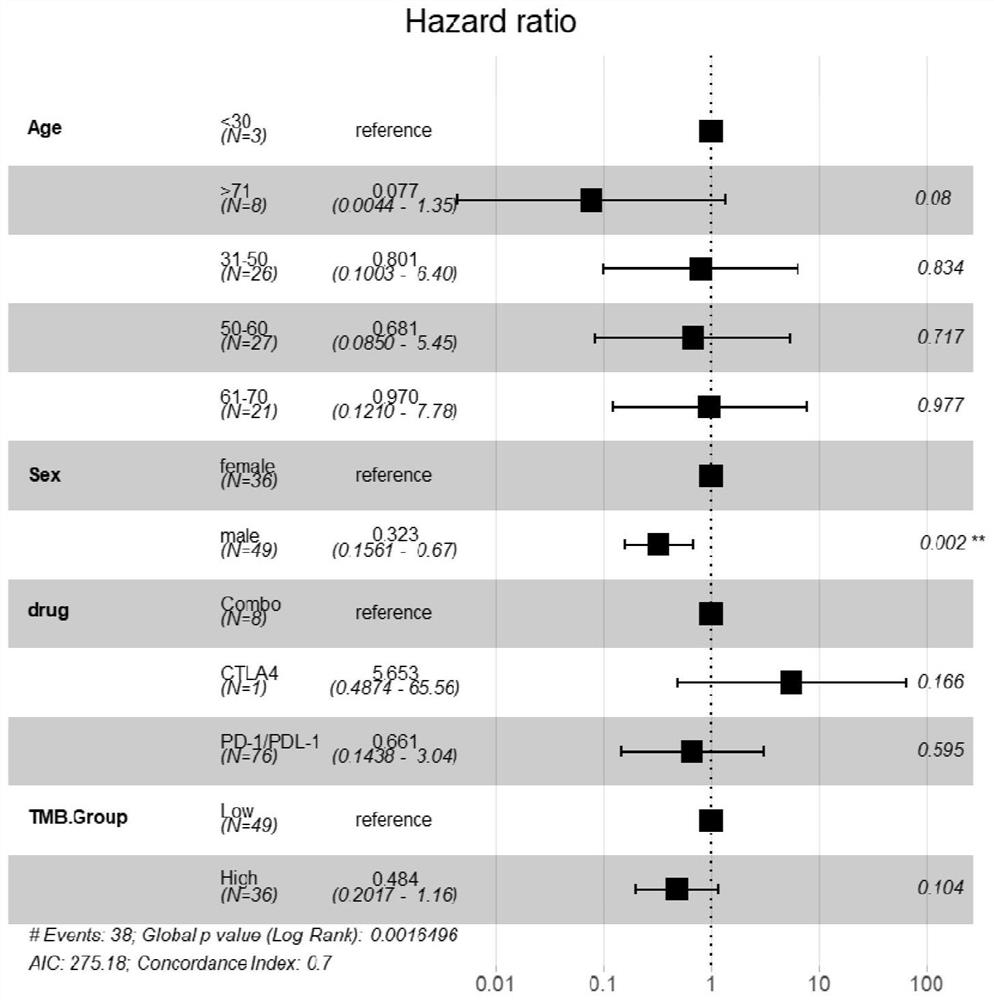
[0105] In order to further verify that the APC gene and FAT1 gene of the present invention are independent predictive risk factors for the efficacy of immunotherapy, the present invention downloads the data of 619 patients with advanced colon adenocarcinoma (COAD) in the DFCI public database, which includes gene mutation data, clinical And survival data, among them, 529 patients had MSI detection results, and found that the somatic mutation status of APC+FAT1 gene was significantly correlated with MSI and TMB status, as shown in Table 3, Table 4 and Table 5.
[0106] table 3
[0107]
[0108] Table 4
[0109] group MSS MSI MSI ratio GS=0 264 21 7.4% GS=1 174 70 28.7%
[0110] table 5
[0111] group TMB-High TMB-Low TMB-High Ratio GS=0 25 302 7.1% GS=1 89 203 30.5%
[0112] The samples with somatic mutation in APC gene were classified as APC=1 group; otherwise, the samples without any somatic mutation...
Embodiment 3
[0117] This embodiment provides a method for predicting the sensitivity of colorectal cancer patients to immunotherapy, comprising the following steps:
[0118] S1: Obtain the clinicopathological information of the subject, and determine whether the subject is a patient with advanced colon adenocarcinoma;
[0119] S2: If the subject is a patient with advanced colon adenocarcinoma, obtain the mutation data of the subject's APC and FAT1 genes, and judge whether there are mutations in the APC gene and the FAT1 gene based on the mutation data;
[0120] S3: If there is a mutation in the APC gene and there is no mutation in the FAT1 gene, calculate GS=0, otherwise calculate GS=1;
[0121] S4: Output the prediction result of the subject's sensitivity to immunotherapy according to the GS score, output the prediction result of the subject's sensitivity to immune checkpoint inhibitor therapy when GS=1, and output the prediction result of the subject's sensitivity to immune checkpoint in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com