Application of nicotinamide compound in preparation of medicine for treating herpes simplex encephalitis
A nicotinamide, herpes simplex technology, applied in the field of medicine, can solve the problems of mutagenicity, drug resistance, seriousness, etc., and achieve the effects of improving the symptoms of encephalitis, inhibiting the infection of encephalitis, and weakening the inflammatory response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1 Niacinamide, nicotinic acid, nicotinamide nitrogen oxide comprehensive antiviral ability in vitro
[0031] The structural formulas of nicotinamide (compound 2, Nicotinamide), nicotinic acid (compound 5, Nicotinic acid), and nicotinamide nitrogen oxide (compound 1, Nicotinamide N-oxide) are as follows:
[0032]
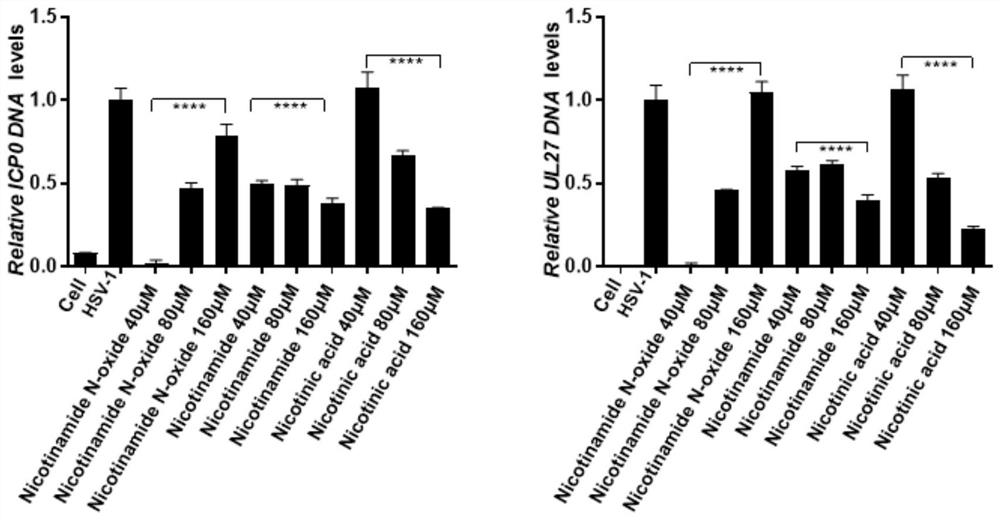
[0033] Will 2×10 5 N2a was inoculated in 12-well plates, which were placed in 5% CO 2 , Cultivated overnight in a cell incubator at 37°C; the cells were divided into blank control group (cell), virus control group (HSV-1), nicotinamide-treated virus group (40 μM, 80 μM and 160 μM), niacin-treated virus group (40 μM , 80 μM and 160 μM) and nicotinamide nitrogen oxide treated virus groups (40 μM, 80 μM and 160 μM), after 12 hours of co-treatment with the drug and the virus, the viral DNA was extracted to detect the effect of the drug on the copy number of the viral DNA level, and the results were as follows figure 1 shown;
[0034] Depend on figur...
Embodiment 2
[0035] Example 2 Niacinamide, nicotinic acid, and nicotinamide nitrogen oxide inhibit the adsorption process of viruses
[0036] The structural formulas of nicotinamide (compound 2, Nicotinamide), nicotinic acid (compound 5, Nicotinic acid), and nicotinamide nitrogen oxide (compound 1, Nicotinamide N-oxide) are as follows:
[0037]
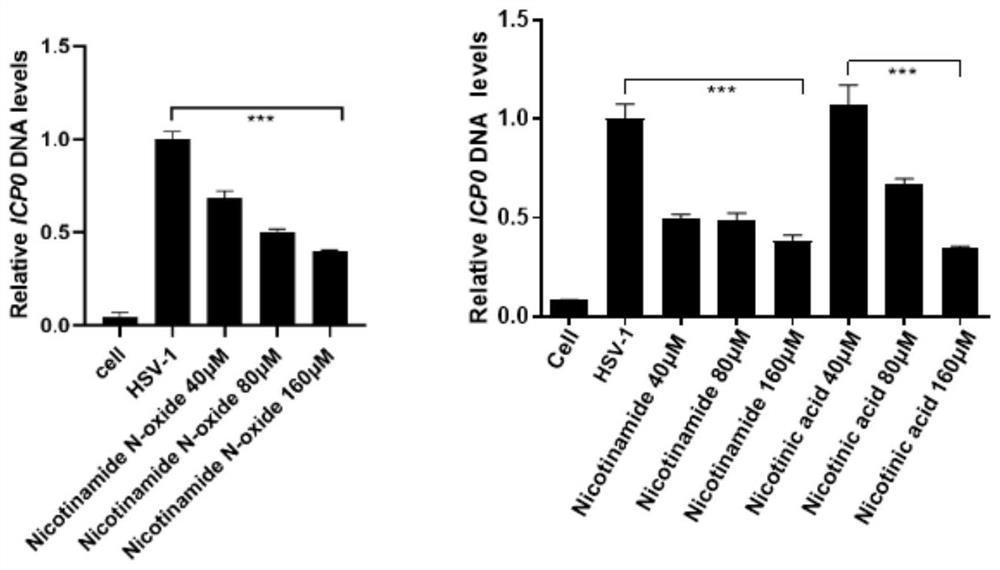
[0038] Will 2×10 5 N2a was inoculated in a 12-well plate, and the culture plate was placed in 5% CO 2 , Cultivated overnight in a cell incubator at 37°C; the cells were divided into blank control group (cell), virus control group (HSV-1), nicotinamide-treated virus group (40 μM, 80 μM and 160 μM), nicotinic acid-treated virus group (40 μM , 80 μM and 160 μM) and nicotinamide nitrogen oxide treatment virus group (40 μM, 80 μM and 160 μM), the culture plate was placed at 4 ° C, after the drug and virus were co-incubated for 80 min, the supernatant was discarded, 500 μL of medium was added, and placed in 5%CO 2 , 37 ℃ cell incubator to continue...
Embodiment 3
[0040]Example 3 Niacin prevents HSV-1 infection test
[0041] The structural formula of nicotinamide (compound 2, Nicotinamide) is as follows:
[0042]
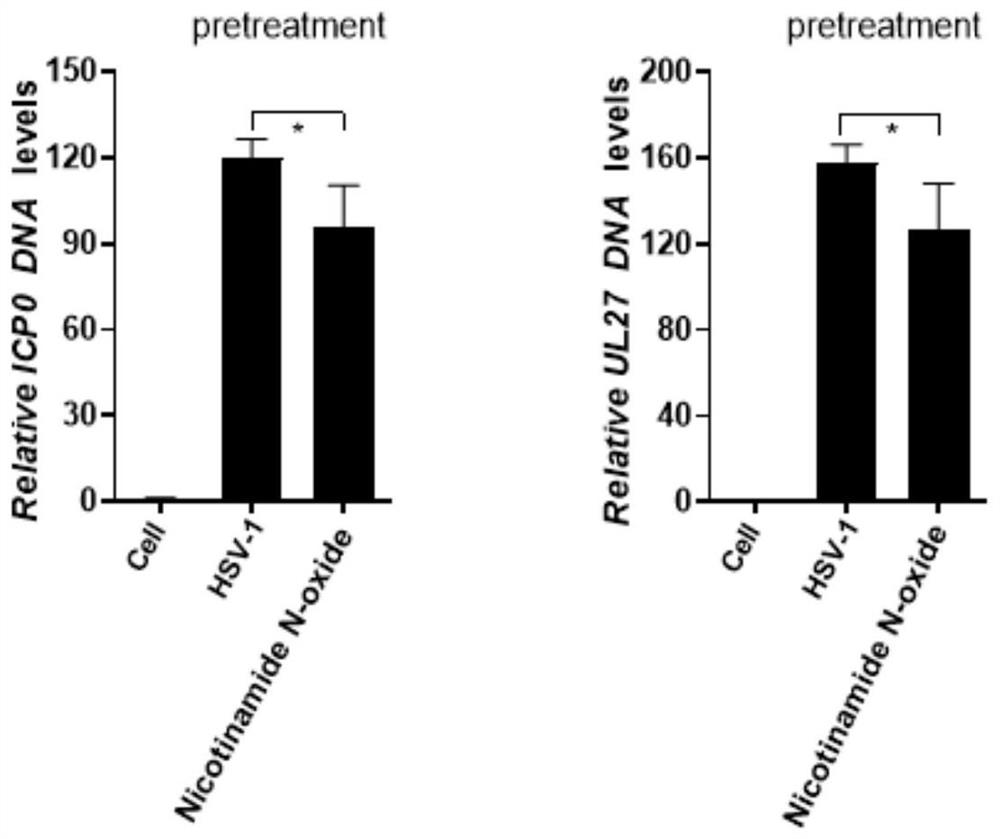
[0043] Will 2×10 5 N2a was inoculated in a 12-well plate, and the culture plate was placed in 5% CO 2 , Cultivated overnight in a 37°C cell incubator; the cells were divided into blank control group (cell), virus control group (HSV-1), and nicotinamide nitrogen oxide (40 μM) pretreated virus group; 40 μM nicotinamide nitrogen oxide The pretreatment virus group was pretreated with drugs for 3 hours, and then the drugs were discarded. After 12 hours of virus infection, the virus DNA was extracted and the copy number of the virus DNA was detected. The results are as follows: image 3 shown;
[0044] Depend on image 3 The results showed that compared with the virus group, the viral DNA copy number of the virus group treated with 40 μM nicotinamide nitrogen oxide was significantly reduced, indicating that this type of drug...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com