S100A8 as blood biomarker for non-invasive diagnosis of endometriosis
An endometriosis, S100A8 technology, applied in disease diagnosis, biological testing, biological material analysis, etc., can solve problems such as limited diagnostic utility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0074] In a first aspect, the present invention relates to a method of assessing whether a patient has endometriosis or is at risk of developing endometriosis comprising
[0075] a) determining the amount of S100A8 in a sample from said patient, and
[0076] b) Comparing the determined amount with a reference.
[0077] In an embodiment, an elevated amount of S100A8 in a sample from a patient is indicative of the presence or risk of developing endometriosis in the patient. In particular, if the amount of S100A8 in the patient's sample is higher than the reference or the amount of S100A8 in the reference sample, the amount of S100A8 in the patient's sample is indicative of the presence or risk of developing endometriosis in the patient. risk. In particular, in assessing the presence of endometriosis or at risk of developing endometriosis compared to the same fluid sample from individuals not suffering from endometriosis or at risk of developing endometriosis Higher amounts of...
example 1
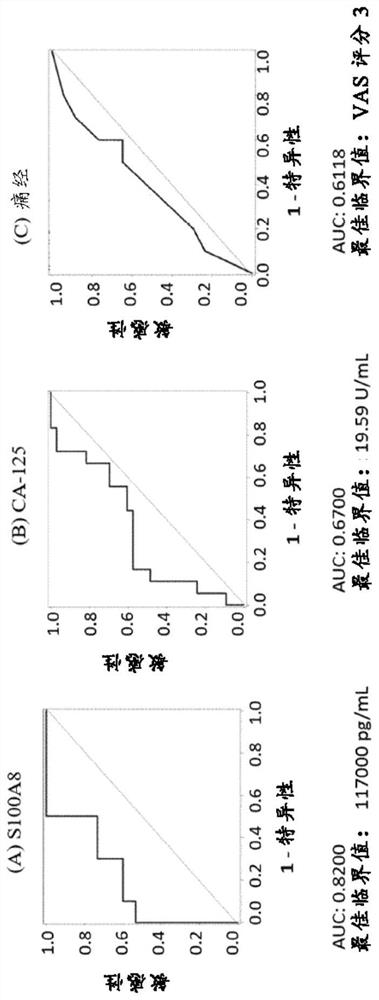
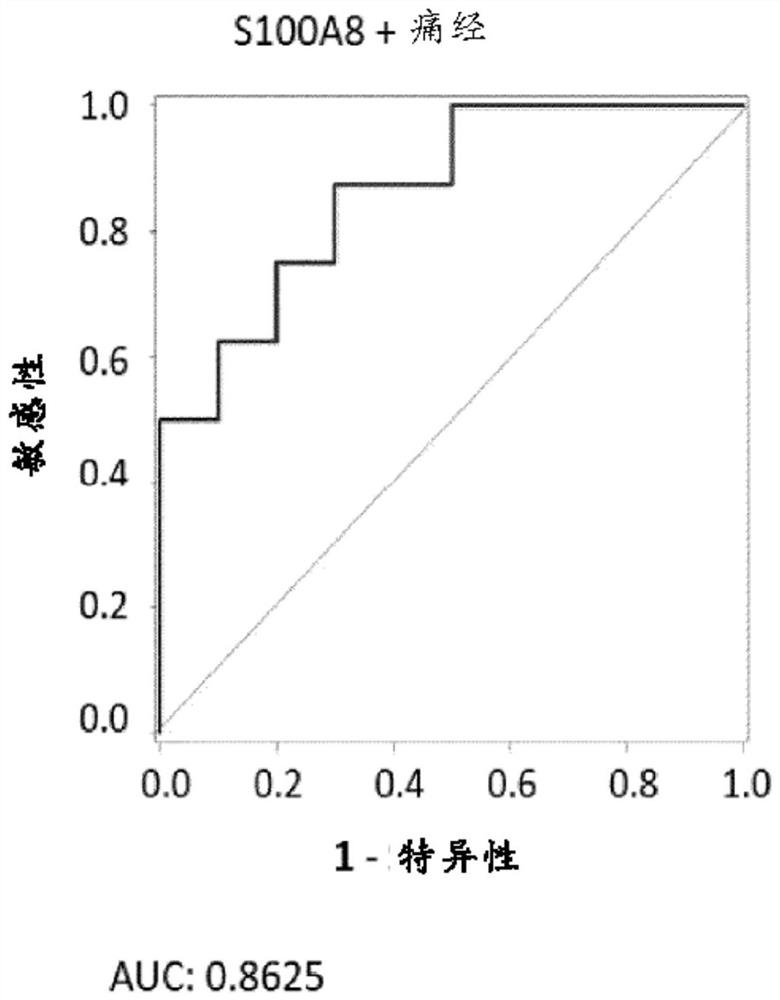
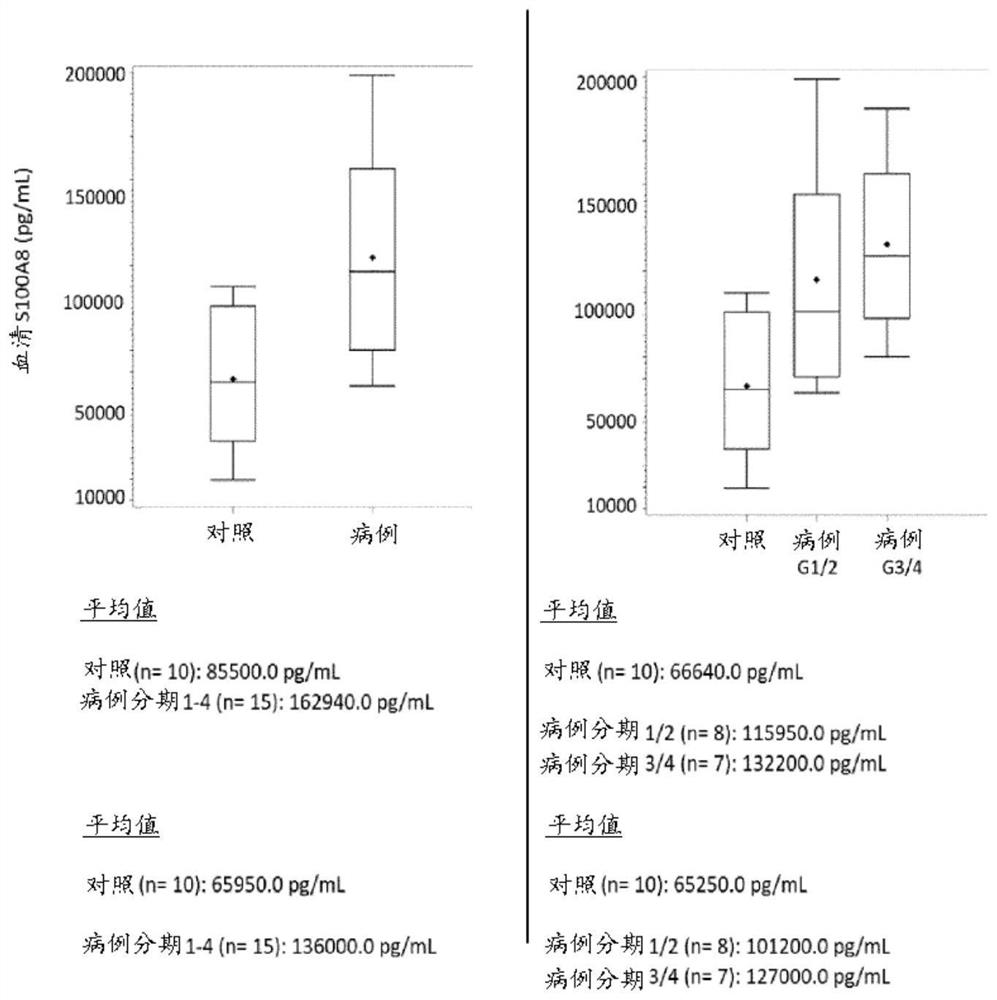
[0168] Example 1: Diagnostic performance of biomarker S100A8 and biomarker combinations in endometriosis and control women
[0169] For the measurements, a total of 21 serum and 31 plasma samples from human females were analyzed. Analyte concentrations were determined by ELISA (Enzyme-Linked Immunosorbent Assay). The case group consisted of patients with laparoscopy-diagnosed pelvic endometriosis (ASRM stages I-IV), and the control group included healthy women without endometriosis.
[0170]The concentration of S100A8 in human serum was determined using the human S100A8 / MRP8 ELISA kit from CircuLex / MBL (distributed by Biozol Eching, Germany; catalog number: CY-8061). The kit utilizes quantitative sandwich ELISA technology. The measurement range of this assay is 78.1 pg / mL-5,000 pg / mL. Microtiter plates were pre-coated with a monoclonal antibody specific for human S100A8. Samples were measured at 200-fold dilutions. After bringing all reagents to room temperature, add 100 ...
example 2
[0185] Example 2 Diagnostic performance of biomarker S100A8 and biomarker combinations in adenomyosis and control women.
[0186] The case group consisted of patients diagnosed with adenomyosis by laparoscopy with subsequent histological confirmation, and the control group consisted of healthy women without adenomyosis. Inclusion criteria for the case group were presence of pelvic pain / infertility and age between 18-45 years. The exclusion criteria for the case group were pregnancy / lactation, malignancy, recurrence of adenomyosis, and laparoscopy / laparotomy ≤6 months.
[0187] Concentrations of S100A8 in human serum were determined using the human S100A8 / MR8 ELISA kit from CircuLex / MBL (see Example 1 above for details).
[0188] Concentration of CA-125 was used by cobas e 601 analyzer CA 125 II was determined as previously described in Example 1.
[0189] Receiver operating characteristic (ROC) curves were generated from univariate models of single biomarkers. Model perfo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com