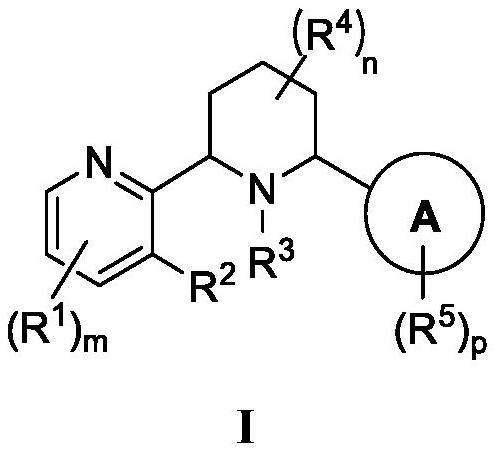
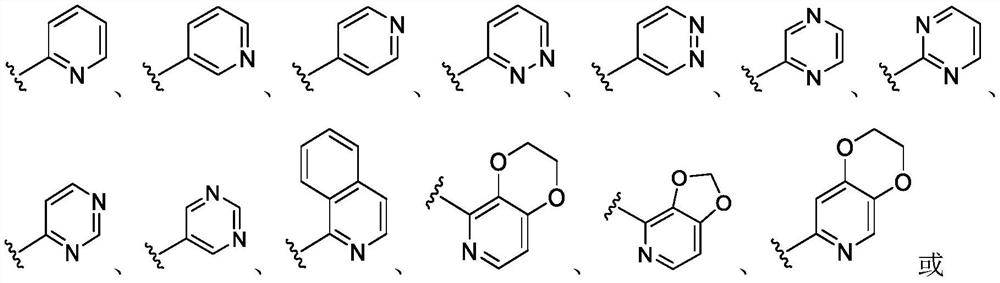
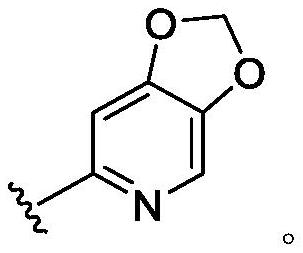
CXCR4 inhibitors and uses thereof
An unsaturated, independent technology, applied in the field of CXCR4 inhibitors and its use, can solve the problems of deletion, loss of response, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0462] Example 1: Synthesis of I-9
[0463] Synthetic scheme of I-9
[0464]
[0465] Synthesize 1-0.
[0466]
[0467] 3-Methyl picoline aldehyde (1-a; 10.0g, 82.55mmol), acetone (60mL) and K 2 CO 3 (17.11g, 123.83mmol) in toluene-EtOH-H2 The mixture in O solvent (150 mL+60 mL+30 mL) was stirred at 70 °C for 16 hours. After cooling to rt, the solvent was evaporated in vacuo. The resulting residue was partitioned between DCM and H 2 Between O. The aqueous phase was further extracted twice with DCM. The combined organic phases were washed with brine and washed with anhydrous Na 2 SO 4 Dry and filter. The filtrate was concentrated in vacuo and the residue was purified by column chromatography to obtain 1-0 (9.20 g, 69.1%) as a light green solid. LCMS (Agilent LCMS 1200-6120, column: Waters X-Bridge C18 (50mm * 4.6mm * 3.5 μ m); Column temperature: 40 ℃; Flow rate: 2.0 milliliters / minute; Mobile phase: within 1.6 minutes 90% [( Total 10mM AcONH 4 ) water / CH 3...
example 2
[0483] Example 2: Synthesis of I-1, I-5, I-6 and I-7
[0484] Synthetic scheme of I-1, I-7, I-5 and I-6
[0485]
[0486] Synthesis 2-1.
[0487]
[0488] 2-0 (9g, 41.66mmol), palladium diacetate (935mg, 4.17mmol), P (o-tolyl) 3 (2.5g, 8.34mmol), acrylonitrile (22g, 416.60mmol) and triethylamine (12g, 125mmol) in DMF (20mL) was stirred in a sealed tube at 130°C for 4 hours. Then, the suspension was filtered; the filtrate was poured into water and extracted with dichloromethane (50 mL×3), the separated organics were concentrated in vacuo and the residue was purified by column chromatography (PE / EA=10 / 1) to give 2-1 (2 g, yield: 26%). LCMS (Agilent LCMS 1200-6120, column: Waters X-Bridge C18 (50mm * 4.6mm * 3.5μm); Column temperature: 40 ℃; Flow rate: 2.0 ml / min; Mobile phase: 90% [water in 0.5 minutes +10mM NH 4 HCO 3 ] and 10% [CH 3 CN] to 5% [water + 10mM NH 4 HCO 3 ] and 95% [CH 3 CN], then continued under these conditions for 1.5 minutes, and finally becam...
example 3
[0513] Example 3: Synthesis of I-2 and I-3
[0514] Synthetic scheme of I-2 and I-3
[0515]
[0516] Synthetic schemes of I-2 and I-3 (continued)
[0517]
[0518] Synthesis 3-2.
[0519]
[0520] 3-0 (10.0g, 46.3mmol), 3-a (13.6g, 138.9mmol), CuI (1.8g, 9.3mmol), Pd(PPh 3 ) 2 Cl 2 (3.2g, 4.6mmol) and DIPEA (25mL) in THF (250mL) mixture in N 2 Stirring was carried out at 60° C. for 2 hours under an atmosphere. After LCMS indicated that the reaction was complete, the mixture was cooled to room temperature, treated with TBAF (56 mL, 1 M) in THF and stirred at room temperature for 1 hour. After TLC indicated that the reaction was complete, the reaction mixture was filtered through celite. The filtrate was concentrated in vacuo and the residue was purified by silica column chromatography to afford 3-2 (6.5 g, 87%) as a black solid. LCMS (Agilent LCMS 1200-6120, column: Waters X-Bridge C18 (50mm * 4.6mm * 3.5μm); Column temperature: 40 ℃; Flow rate: 2.0 ml / min; ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com