Sample pad sealing agent as well as preparation method and application thereof
A sample pad and sealing agent technology, applied in the field of medical detection, can solve problems such as interference, achieve the effects of good stability, improve detection specificity and accuracy, and improve anti-interference ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
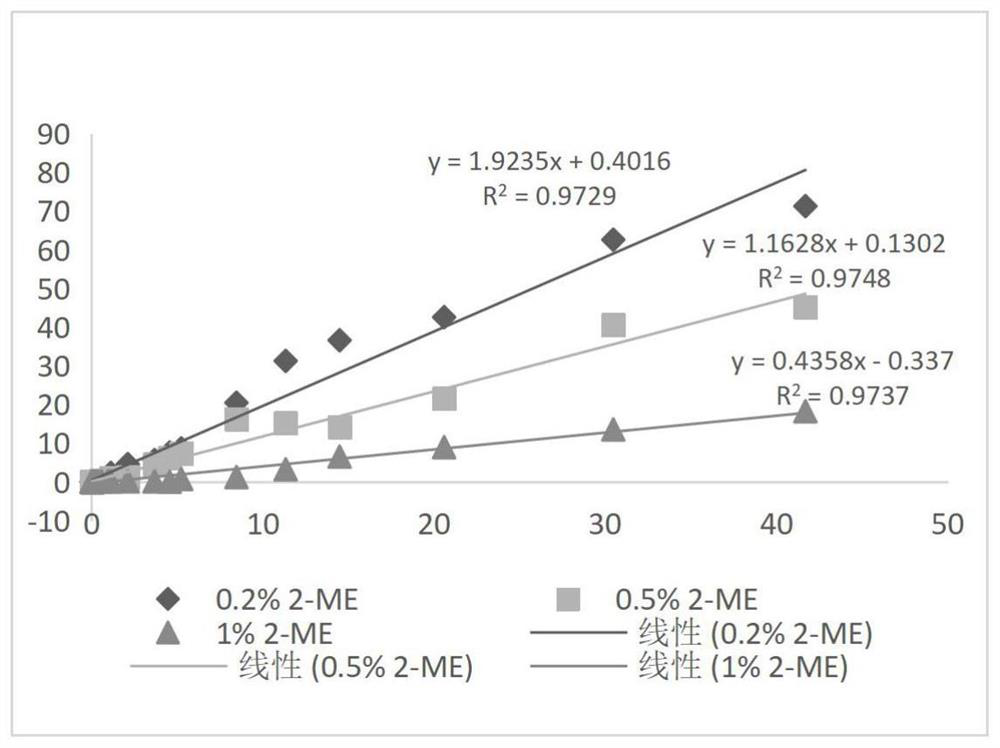
[0050] Example 1: Comparison of different concentrations of 2-ME in blocking agent without adding cleaning antibody composition
[0051] (1) Prepare 100mL 0.5mol / L Tris-HCl buffer solution: weigh 6.057g Tris, dissolve it completely with 70mL purified water, then adjust the pH to 7.48-7.52 with 5mol / L HCl solution, mix well, and use purified Dilute water to 100mL and let stand for use;
[0052] (2) Take a clean beaker, add purified water 60mL, 0.5mol / L Tris-HCl buffer 32mL, trehalose 16g, Tween-200.64mL, Procilin3000.64mL, add purified water to 160mL, and divide into 4 Add 16mg of RBC mab-clon7H3 to it, and then add 0mL, 0.16mL, 0.4mL, 0.8mL of 2-ME to it, mix thoroughly, add purified water to 80mL respectively, and the 4 parts of liquid are referred to as 0% (v / v) 2-ME, 0.2% (v / v) 2-ME, 0.5% (v / v) 2-ME, 1% (v / v) 2-ME;
[0053] (3) Take 45 mL of each of the 4 parts of liquid in step (2) and infiltrate 4 parts of sample pads respectively, and let stand for 5 minutes;
[0054]...
Embodiment 2
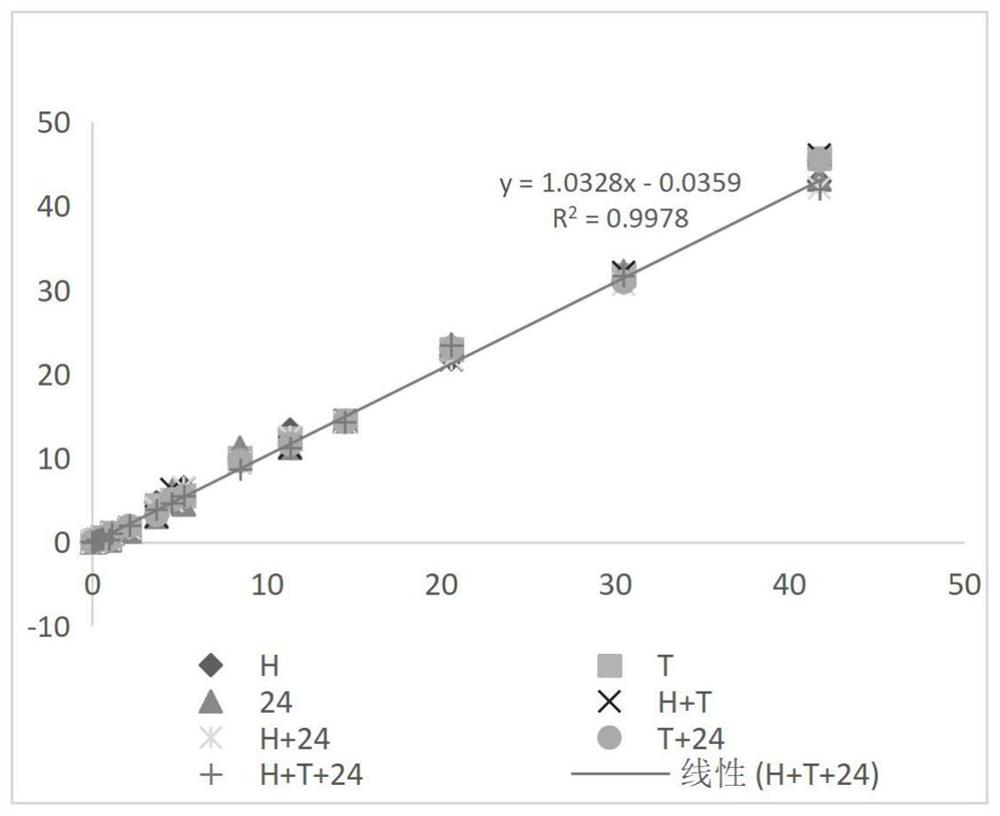
[0085] Example 2: Comparison of Cleaning Antibody Combinations
[0086] (1) Prepare 100mL 0.5mol / L Tris-HCl buffer solution: weigh 6.057g Tris, dissolve it completely with 70mL purified water, then adjust the pH to 7.48-7.52 with 5mol / L HCl solution, mix well, and use purified Dilute water to 100mL and let stand for use;
[0087](2) Add 100mL of purified water to the beaker, add 56mL of Tris-HCl buffer solution in step (1), 28g of trehalose, Tween-201.12mL, Procilin3001.12mL, add purified water to 280mL, and divide into 7 parts, add 0.4mL 2-ME, 16mg RBC mab-clon7H3 to each part, then add 16mg HBR-2, 16mg Tru Block-2, 16mg CAB mab-clon 24, 16mg HBR-2+16mg Tru Block- 2. 16mg HBR-2+16mg CAB mab-clon24, 16mg Tru Block 2+16mg CAB mab-clon 24, 16mg HBR-2+16mg Tru Block-2+16mgCAB mab-clon 24, mix well and add purified water To 80mL, the 7 liquids are referred to as H, T, 24, H+T, H+24, T+24, H+T+24 respectively;
[0088] (3) Take 45mL of each of the 7 parts of liquid in step (2) a...
Embodiment 3
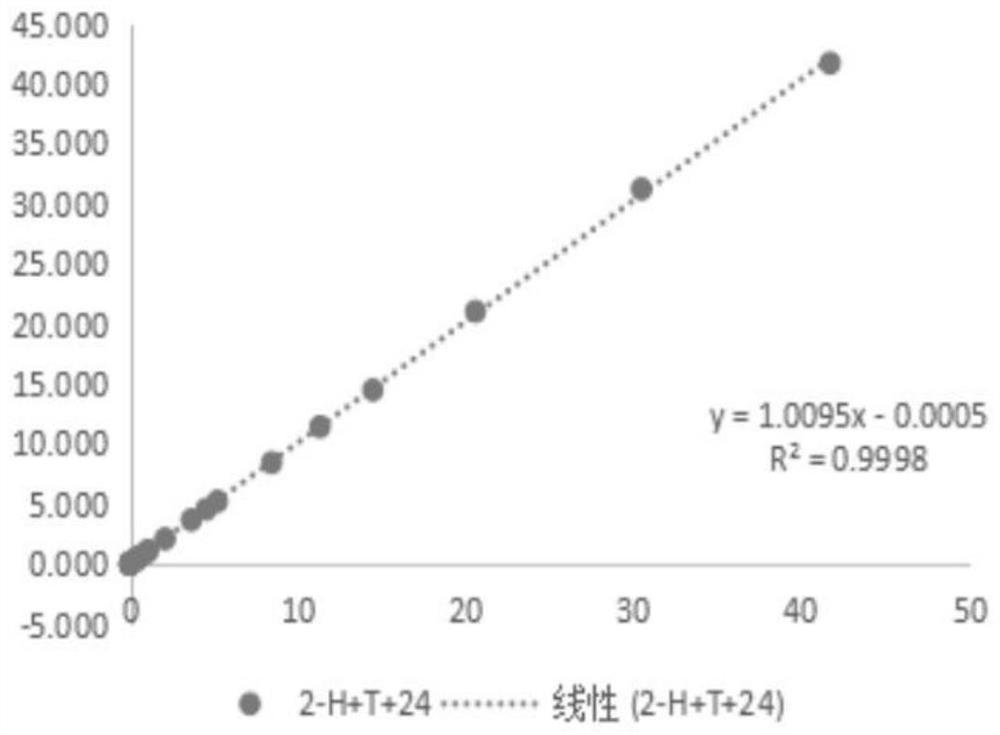
[0109] Example 3: Evaluation of the interaction of reducing agents and cleaning antibodies
[0110] (1) Prepare 100mL 0.5mol / L Tris-HCl buffer solution: weigh 6.057g Tris, dissolve it completely with 70mL purified water, then adjust the pH to 7.48-7.52 with 5mol / L HCl solution, mix well, and use purified Dilute water to 100mL and let stand for use;
[0111] (2) Add 100 mL of purified water to the beaker, add 8 mL of Tris-HCl buffer solution in step (1), 4 g of trehalose, Tween-200.16 mL, Procilin 3000.16 mL, add purified water to 40 mL, and mix well Add 0.4mL 2-ME, 16mg RBC mab-clon7H3, then add 16mg HBR-2+16mg Tru Block-2+16mg CAB mab-clon24, mix well and add purified water to 80mL, referred to as 2-H+T+ twenty four;
[0112] (3) Take 45 mL of the liquid in step (2) and infiltrate 1 part of the sample pad, and let it stand for 5 minutes;
[0113] (4) Put the sample pad soaked in step (3) into an oven set at 37°C for 18 hours;
[0114] (5) Take out the sample pad dried in ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com