Compositions and methods for TCR reprogramming using fusion proteins
A fusion protein and recombinant nucleic acid technology, applied in biochemical equipment and methods, chemical instruments and methods, and the introduction of foreign genetic material using vectors, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
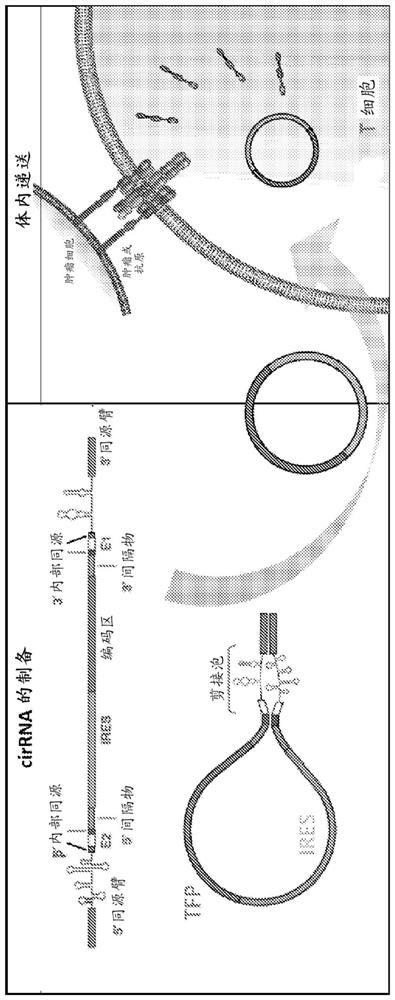
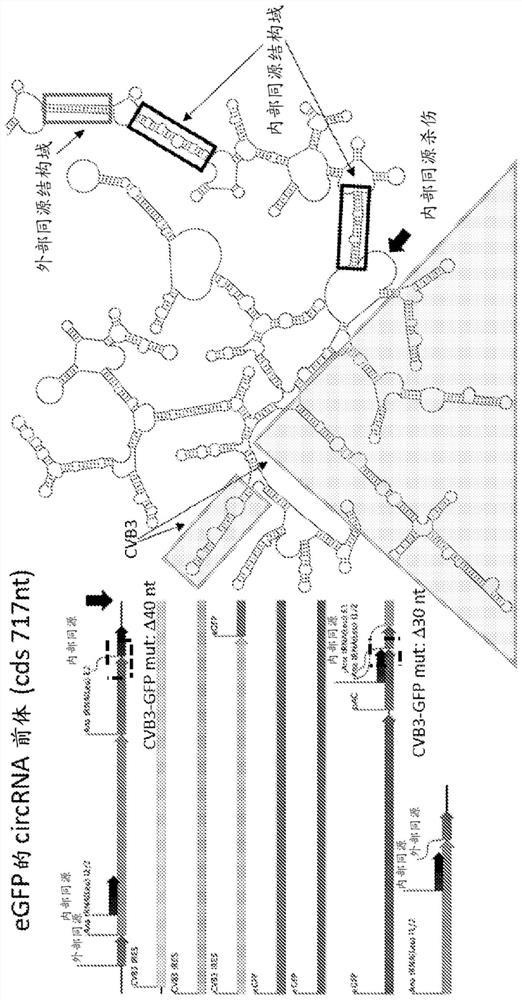
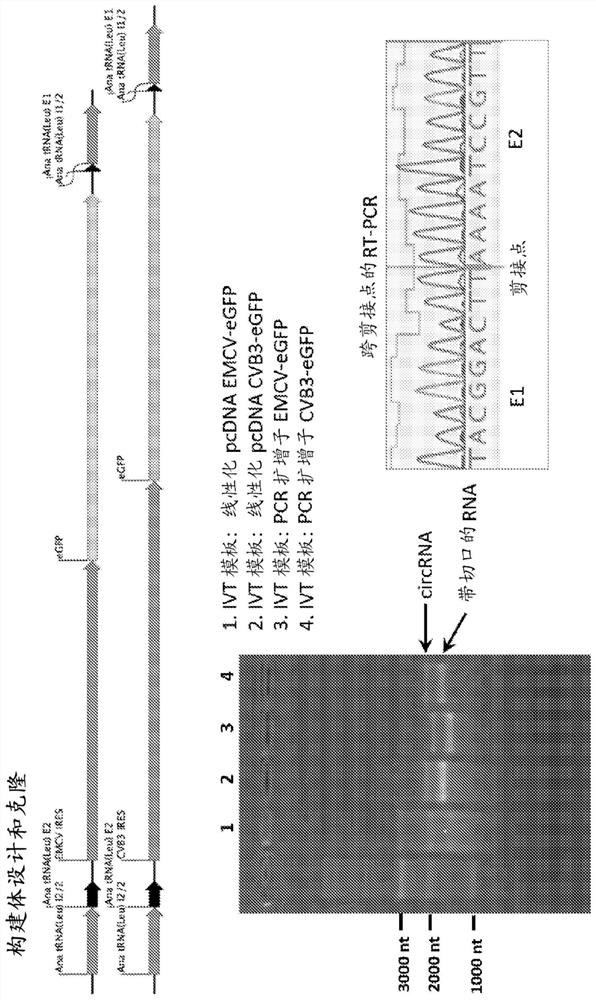
[0389] Example 1: Design of protein-coding circular RNA (circRNA)
[0390] The target protein selected for expression in this experiment was GFP. Translation of functional GFP from circular RNAs is achieved by using ribozymes in a permuted intron-exon (PIE) splicing strategy. To generate a GFP-encoding circRNA, an internal ribosome entry was placed between two short fragments of the E2 and E1 exons downstream and upstream of the group I catalytic intron in the thymidylate synthase (Td) gene from bacteriophage T4 site (IRES), followed by the GFP coding sequence. Alternatively, the E2 and E1 exons downstream and upstream of the group I catalytic intron in the Anabaena precursor tRNA gene can be used, because the splicing efficiency of the group I catalytic intron in the Anabaena precursor tRNA gene is higher than that of bacteriophage T4 The Td gene is more effective. [Puttaraju, M. & Been, M. Nucleic Acids Res. 20, 5357-5364 (1992)]. Finally, the 3' half of the group I cata...
Embodiment 2
[0391] Example 2: Design of circRNA encoding TFP
[0392] Translation of functional TFP from circular RNA (circRNA) can be achieved by using ribozymes in a permuted intron-exon (PIE) splicing strategy. To generate a TFP-encoding circRNA, an internal ribosome entry was placed between two short fragments of the E2 and E1 exons downstream and upstream of the group I catalytic intron in the thymidylate synthase (Td) gene from bacteriophage T4 site (IRES), followed by the TFP coding sequence (CDS). Alternatively, the E2 and E1 exons downstream and upstream of the group I catalytic intron in the Anabaena precursor tRNA gene can be used, because the splicing efficiency of the group I catalytic intron in the Anabaena precursor tRNA gene is higher than that of bacteriophage T4 The Td gene is more effective. [Puttaraju, M. & Been, M. Nucleic Acids Res. 20, 5357-5364 (1992)]. Finally, the 5' half of the group I catalytic intron was cloned upstream of E2, while the 3' half of the group...
Embodiment 3
[0393] Example 3: Design of circRNA encoding CAR and TCR
[0394] The translation of functional CARs, TCRs, from circular RNAs (circRNAs) can be achieved by using ribozymes to align intron-exon (PIE) splicing strategies. To generate circRNAs encoding CAR, TCR, internal ribose was placed between two short fragments of E2 and E1 exons downstream and upstream of group I catalytic intron in the thymidylate synthase (Td) gene from bacteriophage T4 The body entry site (IRES), followed by CAR, TCR coding sequence (CDS). Alternatively, the E2 and E1 exons downstream and upstream of the group I catalytic intron in the Anabaena precursor tRNA gene can be used, because the splicing efficiency of the group I catalytic intron in the Anabaena precursor tRNA gene is higher than that of bacteriophage T4 The Td gene is more effective. [Puttaraju, M. & Been, M. Nucleic Acids Res. 20, 5357-5364 (1992)]. Finally, the 5' half of the group I catalytic intron was cloned upstream of E2, while the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com