Immunostimulatory multimer binding molecules
A technology combining molecules and polymers, applied in the field of p|, can solve problems such as toxicity limitations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
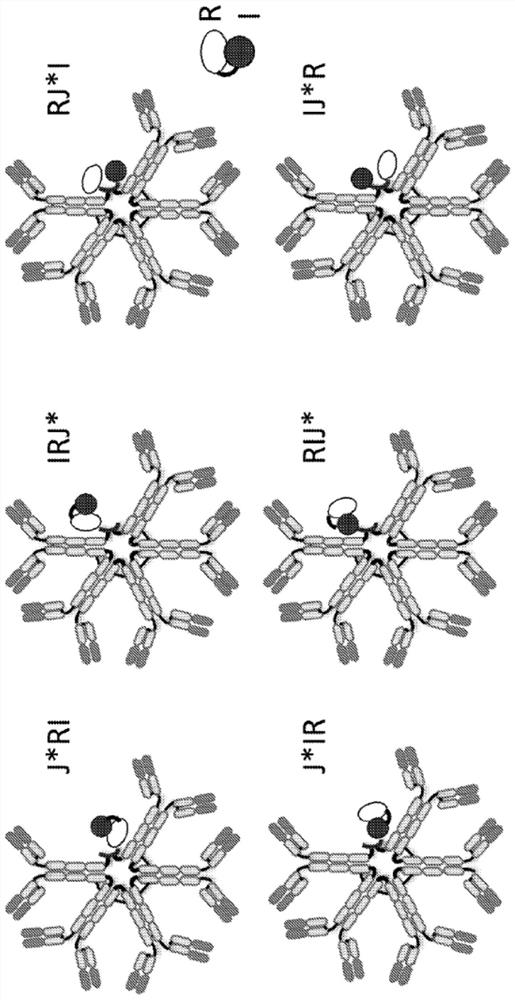
[0216] Example 1: Constructs and Characterization of IgM-Based Immunomodulators (ISAs) with Modified J Chains Expressing IL-15 and IL-15Rα sushi Domains
[0217] For the production of IgM-based immunomodulators (ISAs), a gene expressing the sushi domain of mature human IL-15 (SEQ ID NO: 4) and human IL-15Rα (SEQ ID NO: 5) as a fusion protein was constructed and characterized as follows. Modified J chain. The starting point for the modified J chain is a variant of the mature human J chain comprising a Y to A amino acid substitution at position 102 ("Y102A" or "J*", the amino acid sequence of the variant is presented as SEQ ID NO :3), this substitution enhances the serum half-life of the IgM pentamer comprising the J chain variant. See PCT Publication No. WO 2019 / 169314A1. Initially, IgM antibodies comprising an antigen-binding domain that binds PD-L1 were combined with various fusion proteins comprising all three domains (J*, mature IL-15 (“I”), and IL-15Rα sushi domain) as ...
Embodiment 2
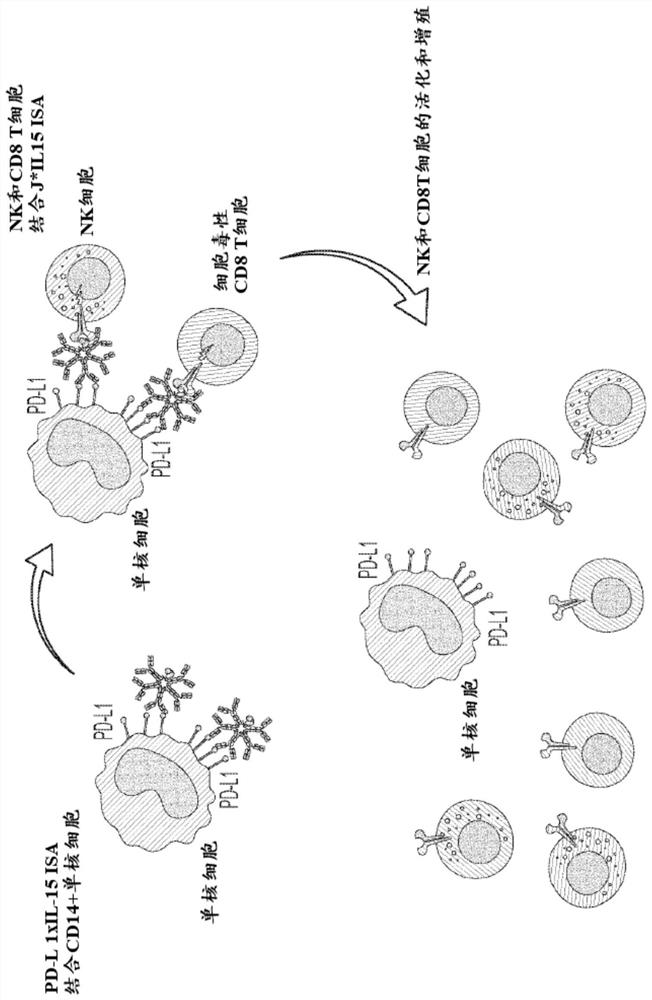
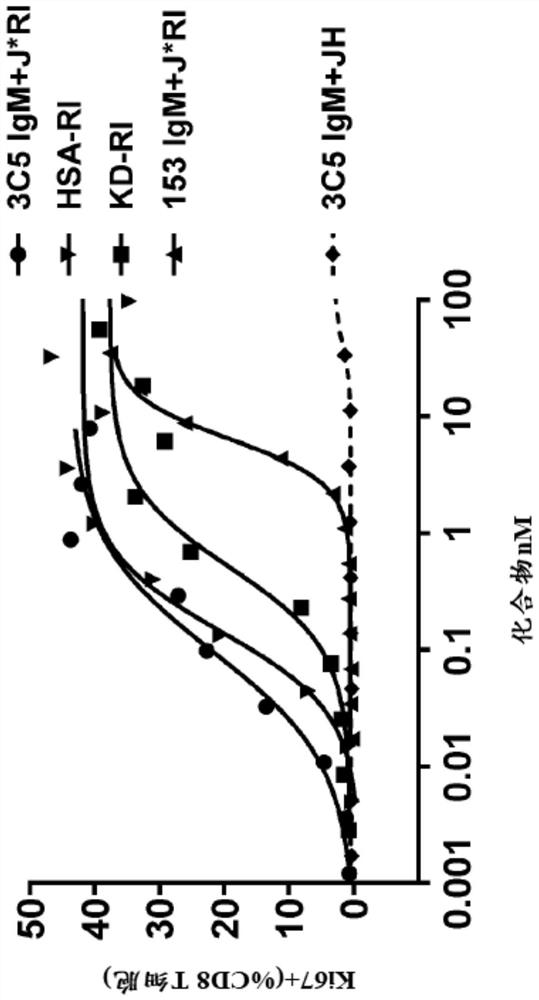
[0225] Example 2: Ki-67 In Vitro Potency Assay of IgM-Based ISA
[0226] The in vitro potency of the various IgM J*RI ISA constructs prepared in Example 1 was assessed in the Ki-67 proliferation assay as follows. This assay measures the proliferation of primary cells (huPBMC, human peripheral blood mononuclear cells) in response to IL-15. Binding of IL-15 to its receptor results in cell proliferation, which can be visualized by several techniques, one of which is the cell cycle-associated protein assay. The most commonly used cell cycle-related protein is Ki-67, which is only expressed in G1, S, G2 and M phases. Determination of Ki-67 protein levels in the nuclei of cytotoxic CD8 T cells and natural killer NK cells (cells physiologically expressing the β and γ subunits of the IL-15 receptor) by flow cytometry is used for actual cell proliferation Surrogate assay. figure 2 A schematic diagram of the assay is shown in .
[0227] Briefly, healthy donor PBMCs are incubated fo...
Embodiment 3
[0236] Example 3: Evaluation of ISAs comprising IL-15 variants with reduced receptor binding
[0237] In certain aspects, eg, to manage the potential toxicities of a therapeutic ISA, it may be desirable to modify the potency of the IL-15 ISA by reducing binding to the IL-15β / γ receptor. Nine residues in mature human IL-15 (SEQ ID NO: 4) were previously identified by others as having the ability to reduce receptor binding, see PCT Publication No. WO 2018 / 071918A1. These include IL-15N1D (SEQ ID NO: 57), N4D (SEQ ID NO: 58), D8N (SEQ ID NO: 59), D30N (SEQ ID NO: 60), D61N (SEQ ID NO: 61), E64Q (SEQ ID NO: 62), N65D (SEQ ID NO: 63), N72D (SEQ ID NO: 64) and Q108E (SEQ ID NO: 65). These mutated IL-15 sequences were incorporated with double mutations N4D / N65D (SEQ ID NO: 66) and N1D / N65D (SEQ ID NO: 67) and triple mutations D30N / E64Q / N65D (SEQ ID NO: 68) into the Modified J chain in J*RI configuration, resulting in a fusion protein having the sequence SEQ ID No: 7-18. A modified...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com