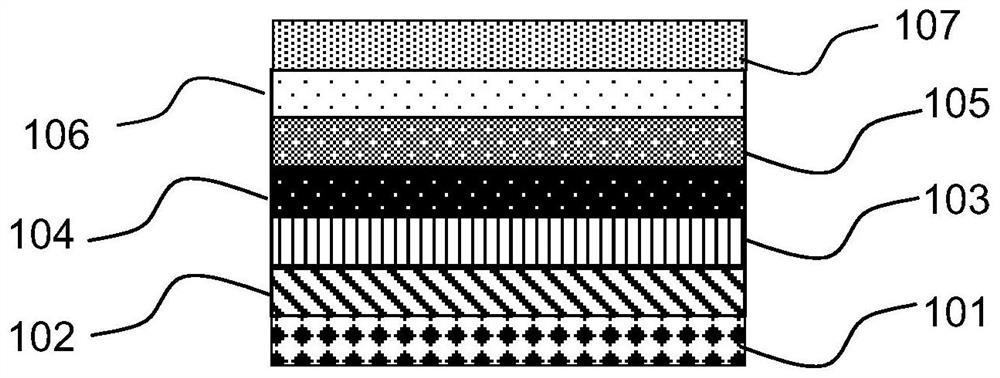
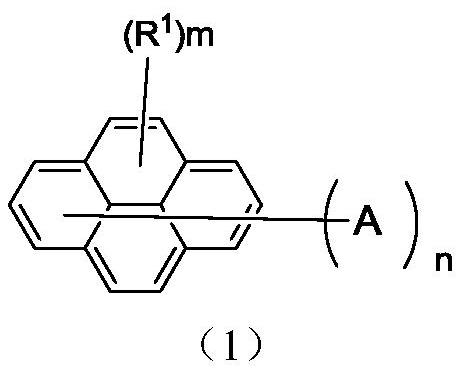
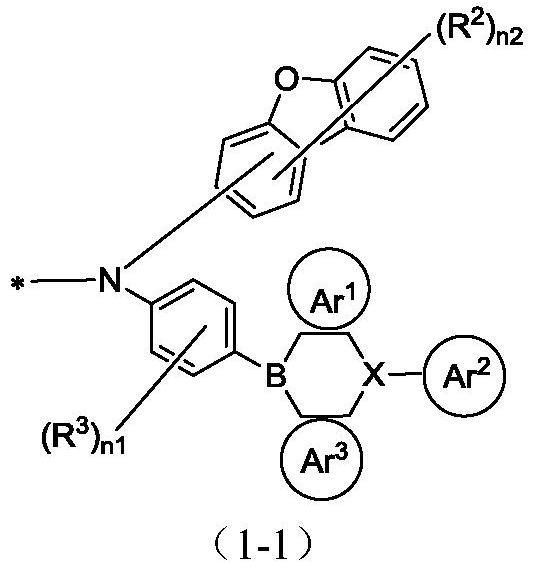
Pyrene organic compound and application thereof
A technology of organic compounds and pyrenes, which is applied in the field of organic electroluminescence, can solve the problems of low luminous efficiency, fast roll-off of efficiency and high cost of luminescent materials, and achieves the improvement of luminous efficiency and life, low manufacturing cost and low roll-off. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0152] The synthetic route of compound (1) is as follows:
[0153]
[0154] Synthesis of Intermediates 1-3:
[0155] Under nitrogen protection atmosphere, in a dry three-necked flask, add 10mmol of intermediate 1-1 and 30mmol of intermediate 1-2 respectively, add 150ml of concentrated sulfuric acid to dissolve it, heat to 80°C until the reaction solution is refluxed, and react for 12 hours , until the reaction is complete, add water to extract the reaction, and simultaneously extract the organic phase with dichloromethane, combine and wash the organic phase several times, dry with anhydrous magnesium sulfate, filter, and spin evaporate to dryness to obtain a crude product. Purified to obtain intermediate 1-3 with a molar weight of 8.65 mmol and a yield of 86.5%. MS (ASAP) = 327.0.
[0156] Synthesis of Intermediates 1-5:
[0157] Under nitrogen protection atmosphere, in a dry three-necked flask, add 1mmol of intermediate 1-3 and 1mmol of intermediate 1-4 respectively, po...
Embodiment 2
[0161] The synthetic route of compound (2) is as follows:
[0162]
[0163]
[0164] Synthesis of Intermediate 2-3:
[0165] Under nitrogen protection atmosphere, in a dry three-necked flask, add 10mmol of intermediate 2-1 and 60mmol of intermediate 2-2 respectively, add 150ml of concentrated sulfuric acid to dissolve it, heat to 80°C until the reaction solution is refluxed, and react for 12 hours , until the reaction is complete, add water to extract the reaction, and simultaneously extract the organic phase with dichloromethane, combine and wash the organic phase several times, dry with anhydrous magnesium sulfate, filter, and spin evaporate to dryness to obtain a crude product. Purified to obtain intermediate 2-3 with a molar weight of 8.36 mmol and a yield of 83.6%. MS (ASAP) = 565.5.
[0166] Synthesis of Intermediates 2-5:
[0167] Under nitrogen protection atmosphere, in a dry three-necked flask, add 1mmol of intermediate 2-3 and 2.5mmol of intermediate 2-4 res...
Embodiment 3
[0171] The synthetic route of compound (3) is as follows:
[0172]
[0173] Synthesis of Intermediate 3-3:
[0174] Under nitrogen protection atmosphere, in a dry three-necked flask, add 10mmol of intermediate 3-1 and 30mmol of intermediate 3-2 respectively, add 150ml of concentrated sulfuric acid to dissolve it, heat to 80°C until the reaction solution is refluxed, and react for 12 hours , until the reaction is complete, add water to extract the reaction, and simultaneously extract the organic phase with dichloromethane, combine and wash the organic phase several times, dry with anhydrous magnesium sulfate, filter, and spin evaporate to dryness to obtain a crude product. The intermediate 3-3 was purified to obtain 8.12 mmol in molar mass, and the yield was 81.2%. MS (ASAP) = 327.0.
[0175] Synthesis of intermediates 3-5:
[0176] Under nitrogen protection atmosphere, in a dry three-necked flask, add 1mmol of intermediate 3-3 and 1mmol of intermediate 3-4 respectively, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com