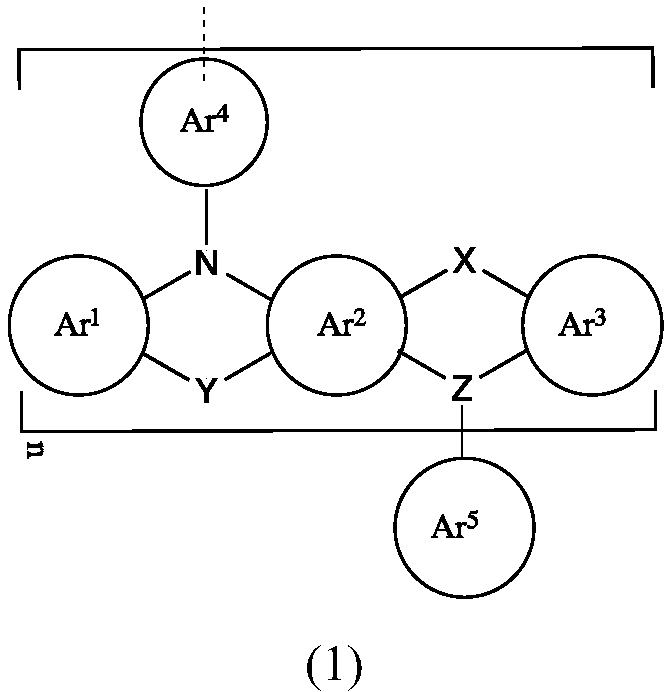
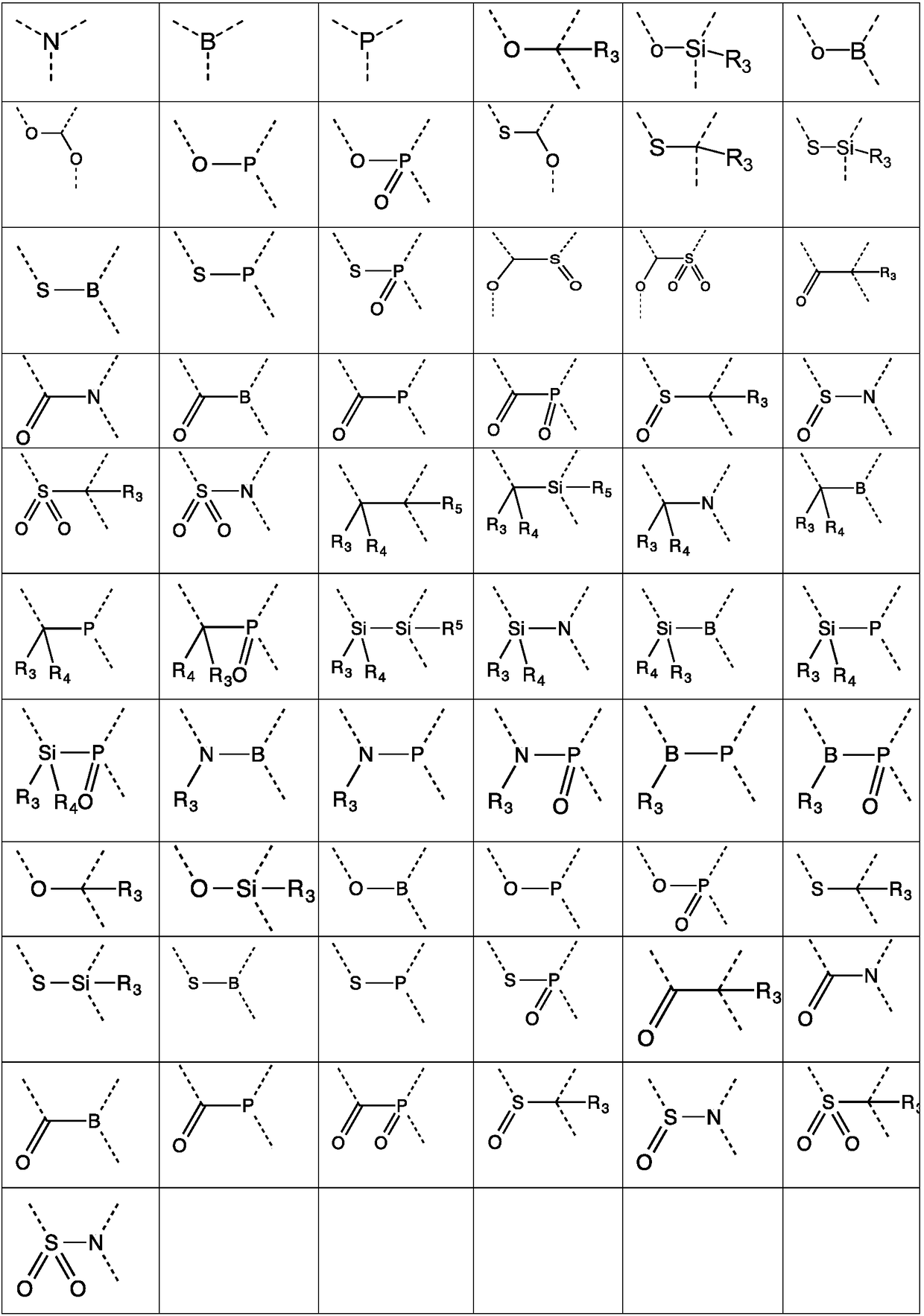
Organic compound, mixture comprising same, composition and organic electronic device
A technology for organic electronic devices and organic compounds, applied in the field of electroluminescent materials, can solve the problems of low fluorescence quantum yield, fast roll-off of efficiency, and low lifespan, and achieve the effects of high efficiency, low manufacturing cost, and long lifespan
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0176]
[0177] 4,4'-bis(5-phenyl-11-indole[3,2-b]carbazolyl)benzene
[0178] Add 2.6g, 10mmol indolo[3,2-b]carbazole, 1.6g, 10mmol bromobenzene, 6.9g, 50mmol potassium carbonate, 0.26g, 1mmol18-crown-6, 0.3g to a 50ml three-necked flask, 1.5mmol cuprous iodide and 100ml o-dichlorobenzene, in N 2 In the atmosphere, react at 140°C, follow the progress of the reaction by TLC, and cool down to room temperature after the reaction is completed. Pour the reaction solution into water, wash to remove K 2 CO 3 , and then suction filtered to obtain a solid product, which was washed with dichloromethane. Recrystallized with ethanol to obtain 3 g of the product 5-phenylindole[3,2-b]carbazole.
[0179] Add 1.6g of the intermediate obtained in the above steps, 5.4mmol 5-phenylindole[3,2-b]carbazole into a 50ml three-necked flask, and simultaneously add 0.65g, 2.5mmol 1,4-dibromobenzene, 3.5g, 25mmol potassium carbonate, 0.13g, 0.5mmol of 18-crown-6, 0.15g, 0.75mmol cuprous iodide an...
Embodiment 2
[0181]
[0182] 4,4’-bis(5-(3,5-diphenylbenzene)-11-H-indole[3,2-b]carbazolyl)terphenyl
[0183] Add 2.6g, 10mmol indolo[3,2-B]carbazole, 3.1g, 10mmol3,5-diphenylbromobenzene, 6.9g, 50mmol potassium carbonate, 0.26g, 1mmol18-crown ether into a 50ml three-necked flask -6, 0.3g, 1.5mmol cuprous iodide and 100ml o-dichlorobenzene, in N 2 In the atmosphere, react at 140°C, follow the progress of the reaction by TLC, and cool down to room temperature after the reaction is completed. Pour the reaction solution into water, wash to remove K 2 CO 3 , and then suction filtered to obtain a solid product, which was washed with dichloromethane. Recrystallized with ethanol to obtain 4 g of the product 5-(3,5-diphenylphenyl)indole[3,2-b]carbazole.
[0184] 2.0g of the intermediate obtained in the above steps, 5.4mmol 5-(3,5-diphenylphenyl)indole[3,2-b]carbazole was added to a 50ml three-necked flask, and 0.89g, 2.5mmol4 -Bromo 4'-iodobiphenyl, 3.5g, 25mmol potassium carbonate, 0.13g,...
Embodiment 3
[0186]
[0187] 5,11-bis(4"-((5-(3,5-diphenylphenyl)indole[3,2-b]carbyl)-11-terphenyl)indole[3,2 -b] carbazole
[0188] In this example, the synthesis steps of the intermediate 5-(3,5-diphenylphenyl)indole[3,2-b]carbazole are exactly the same as those in Example 2.
[0189] The synthesis steps of another intermediate 5,11-bis(4"-bromobitriphenyl)indole[3,2-b]carbazole used in this example are as follows:
[0190] Add 2.6g, 10mmol indolo[3,2-b]carbazole, 8.7g, 20mmol4-bromo-4"-iodo-terphenyl, 6.9g, 50mmol potassium carbonate, 0.26g, 1mmol18- Crown ether-6, 0.3g, 1.5mmol cuprous iodide and 100ml o-dichlorobenzene, in N 2 In the atmosphere, react at 140° C. for 36 hours, track the progress of the reaction by TLC, and cool down to room temperature after the reaction is completed. Pour the reaction solution into water, wash to remove K 2 CO 3 , and then suction filtered to obtain a solid product, which was washed with dichloromethane. Recrystallized with acetone to obtain ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com