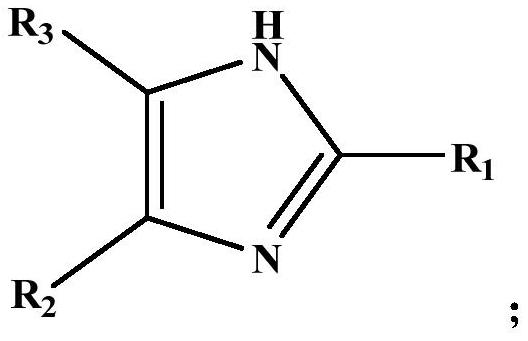
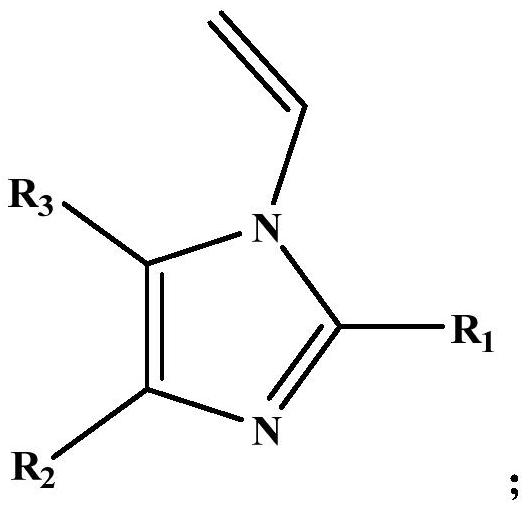
N-vinylation method of alkyl imidazole compound
A technology for alkylimidazoles and compounds, which is applied in the field of N-vinylation of alkylimidazoles to achieve the effects of less by-products, reducing the risk of decomposition and explosion, and avoiding side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Put 900g of 2-methylimidazole and 18g of potassium hydroxide into the reaction, raise the temperature to 150°C, start stirring after the material melts and replace it with nitrogen at the same time. Add acetylene to react, control the reaction pressure in the reactor to 0.7MPaG, stop the reaction after 13h, and obtain the reaction solution containing N-vinylated alkylimidazole compounds. After testing, the reaction solution contained 89.1% of 1-vinyl-2-methylimidazole, 6.5% of 2-methylimidazole, and 4.4% of others (mainly catalysts).
Embodiment 2
[0041] Put 864g of 2-propylimidazole and 73g of sodium hydroxide into the reactor, raise the temperature to 125°C, start stirring after the material melts and replace it with nitrogen at the same time, after 8 hours of replacement, raise the temperature to 150°C (reaction temperature) and keep it, and then pour into the material Feed acetylene to react, control the reaction pressure in the reactor to 0.9 MPaG, and stop the reaction after 15 hours to obtain a reaction solution containing N-vinylated alkylimidazole compounds. After testing, the reaction liquid contained 85.2% of 1-vinyl-2-propylimidazole, 4.7% of 2-propylimidazole, and 10.1% of others (mainly catalyst).
Embodiment 3
[0043] Put 985g of 2-phenylimidazole and 91g of potassium methylate into the reactor, start stirring after raising the temperature to 160°C, vacuum suction (vacuum degree-0.095MPa), raise the temperature to 200°C (reaction temperature) after 3h and keep it, and then add to Pass acetylene into the material for reaction, control the reaction pressure in the reactor to 0.5 MPaG, and stop the reaction after 14 hours to obtain a reaction solution containing N-vinylated alkylimidazole compounds. After testing, the reaction solution contained 84.0% of 1-vinyl-2-phenylimidazole, 5.3% of 2-phenylimidazole, and 10.7% of others (mainly catalyst).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com