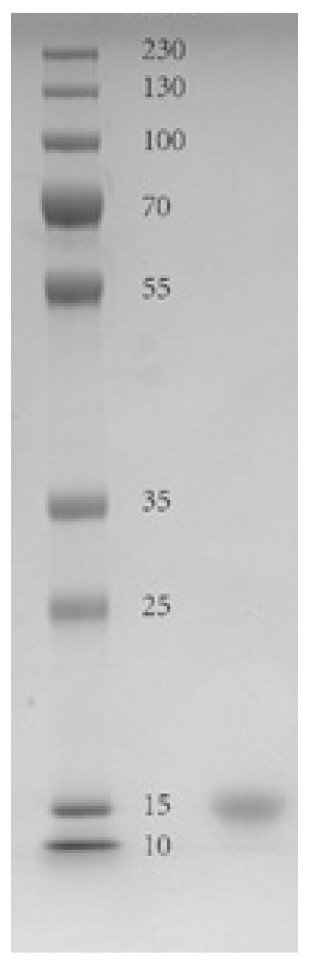
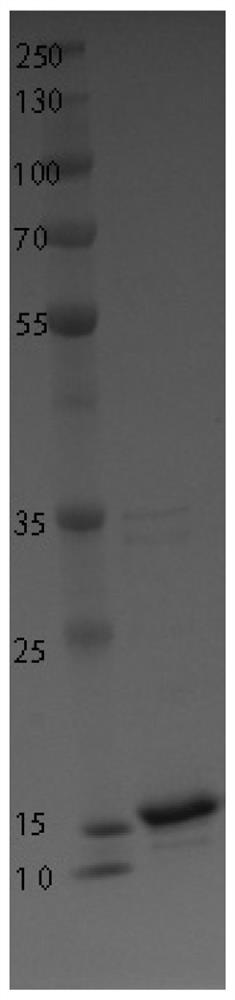
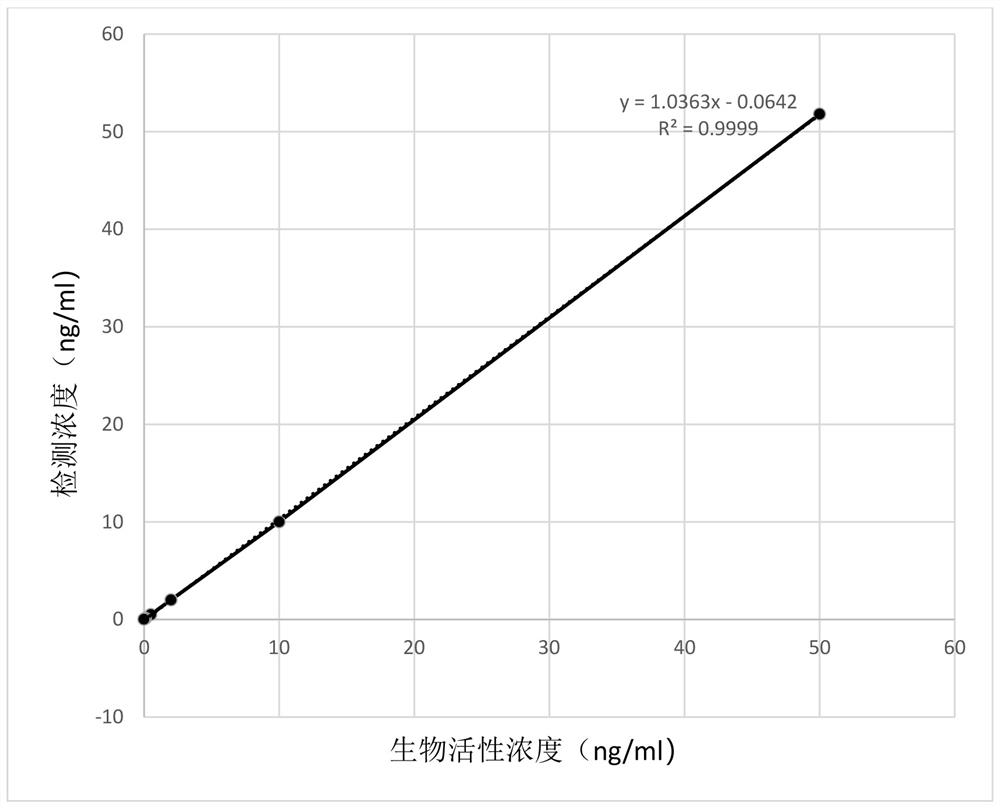
High stability recombinant procalcitonin and its expression vector and application
A technology of procalcitonin and expression vector, applied in the field of protein, can solve problems such as difficulty in meeting requirements, and achieve the effect of good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0028] A first aspect of the present invention, providing:
[0029]A recombinant calcitonin proto-calcitonin, the amino acid sequence is: APFRSALESSPADPATLSEDARLLLAALVQDYVMKASELEQEQEREGSSLDSPRSKKCGNLSTCMLGTYTTQDFNKFHTFPQTAIGVGAPGKKKDMSSDLERDHRPHVSMPQNAN.
[0030] In order to better isolate and purify recombinant proteins, in some examples of recombinant calcitonin protogenes, the C or N-terminus is also linked to a protein isolation tag. After isolation and purification, the isolation label can be further removed by enzymatic lysis. Depending on the characteristics of the separation tag, it can be selected to be connected to the C or N end of the recombinant PCT. Separation labels can be a variety of commonly used separation labels, and there are no special requirements in themselves.
[0031] In some examples of recombinant procalcitonin, the protein isolation tag is ligated via a flexible peptide at the C or N-terminus of the recombinant procalcitonin. This is beneficial for rem...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com