Method for preparing bispecific antibodies
A bispecific antibody and preparation process technology, applied in chemical instruments and methods, specific peptides, peptides, etc., can solve problems such as binding effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0157] Example 1. Design of an IgG1 (Kappa) CH1 and CL domain "cysteine mutation" library
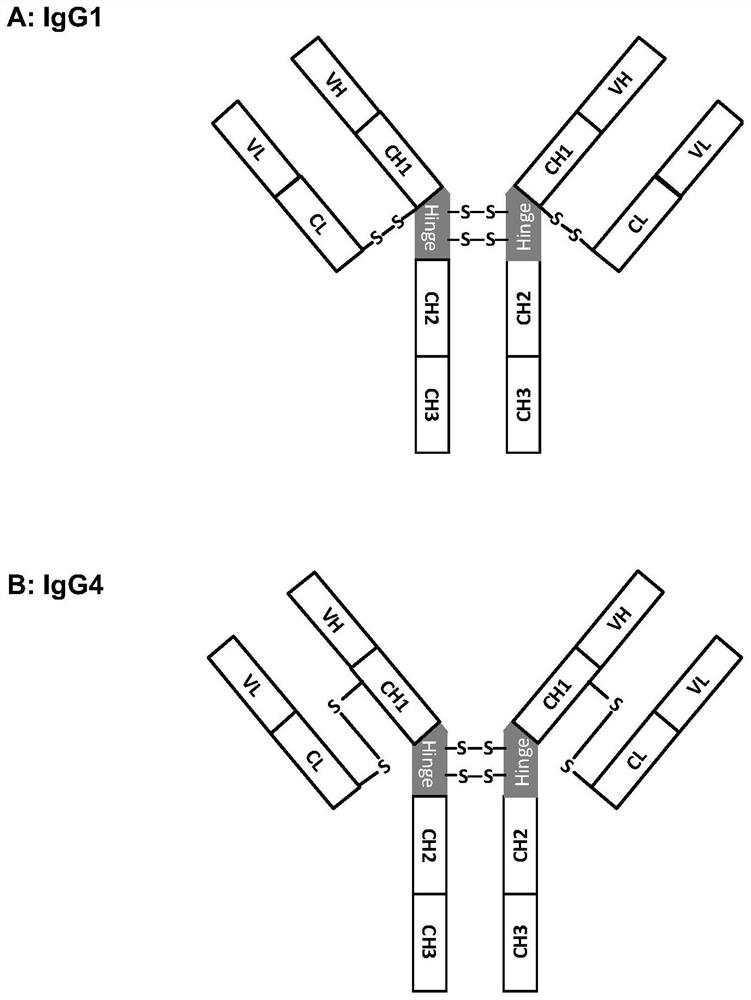
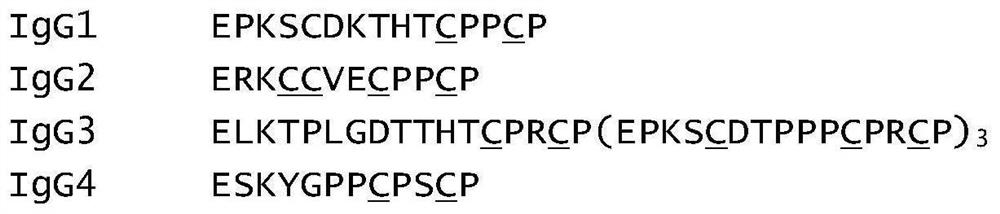
[0158] The CH1 domain sequences of IgG1, IgG2, IgG3 and IgG4 are highly conserved. The light chain is linked to the heavy chain by an interchain disulfide bond in the CH1-CL domain. In the present invention, we hope to replace the natural interchain disulfide bonds with engineered interchain disulfide bonds by mutating the CH1-CL domain of IgG. Using the Fab crystal structure of human IgG1 (kappa) from the Protein Data Bank (PDB code: 1VGE) as a representative structure, cysteine mutations in the CH1 and CL domains that might interact to form interchain disulfide bonds were analyzed and designed location. The cysteine pairs that form natural interchain disulfide bonds are mutated to valine, and new cysteine pairs are introduced at different positions in the CH1-CL domain to form engineered interchain disulfide bonds, by Therefore, an IgG1 (kappa) mutant library was designed. ...
Embodiment 2
[0162] Example 2 Construction and expression of IgG1 (Kappa) "cysteine mutation" library
[0163] Trastuzumab (Trastuzumab, ERBB2 Ab) heavy chain (HoleRF-His) containing T366S / L368A / Y407V "hole" mutations, H435R, Y436F (RF) mutations [EU numbering] and a 6xHis tag at the C-terminus, used as Representative of IgGl (Kappa) heavy chain (SEQ ID NO: 1). Trastuzumab heavy chain (HoleRF-His) codon-optimized DNA was synthesized and used as template for PCR amplification (SEQ ID NO: 2).
[0164]PCR 1, TraH F (SEQ ID NO:3, the primer has an Xho I restriction enzyme site) and TraH R (SEQ ID NO:4, the primer has a Not I restriction enzyme site) primer pair for Trastuzumab PCR amplification of the heavy chain (HoleRF-His) using synthetic DNA as template. The PCR product was digested with Xho I and Not I, and inserted into the same digestion site in pPIC9 (Invitrogen) to construct the expression vector of Trastuzumab heavy chain (HoleRF-His), which was named pPIC9-TraH ( Hole RF-His). ...
Embodiment 3
[0195] Example 3. Screening and identification of IgG1 (Kappa) "cysteine mutation" library
[0196] The concentration of the bispecific antibody in the culture supernatant was measured by ELISA. Briefly, 100 μL / well of 5 μg / mL AGL protein (Leeanntech), 50 mM sodium carbonate buffer (pH 9.6) was added to a 96-well plate (Maxisorp Nunc-Immuno, Thermo Scientific), and coated overnight at 4°C. After washing the plate 3 times with PBS-T (PBS containing 0.05% Tween-20), the plate was blocked with 2% non-fat dry milk in PBS-T for 1 hour at 37°C. After washing the plate 3 times with PBS-T, add 100 μL / well of human IgG standard (initial concentration 2.5ug / ml) and culture supernatant diluted 1:2 in PBS-T, at 37°C Incubate for 1 hour. The plate was washed 3 times with PBS-T, and 100 μL / well of AGL-HRP (Leeanntech) diluted 1:5000 in PBS-T and 0.5% skimmed milk powder was added. The plate was incubated at 37° C. for 1 hour, washed 3 times with PBS-T, and 100 μL / well of TMB (Leeanntec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com