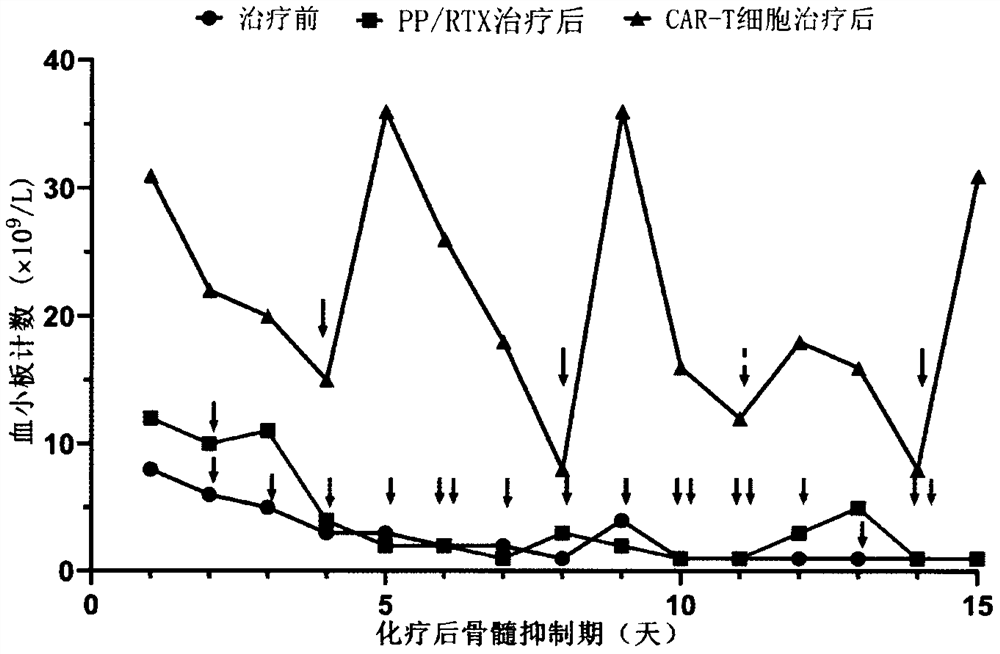
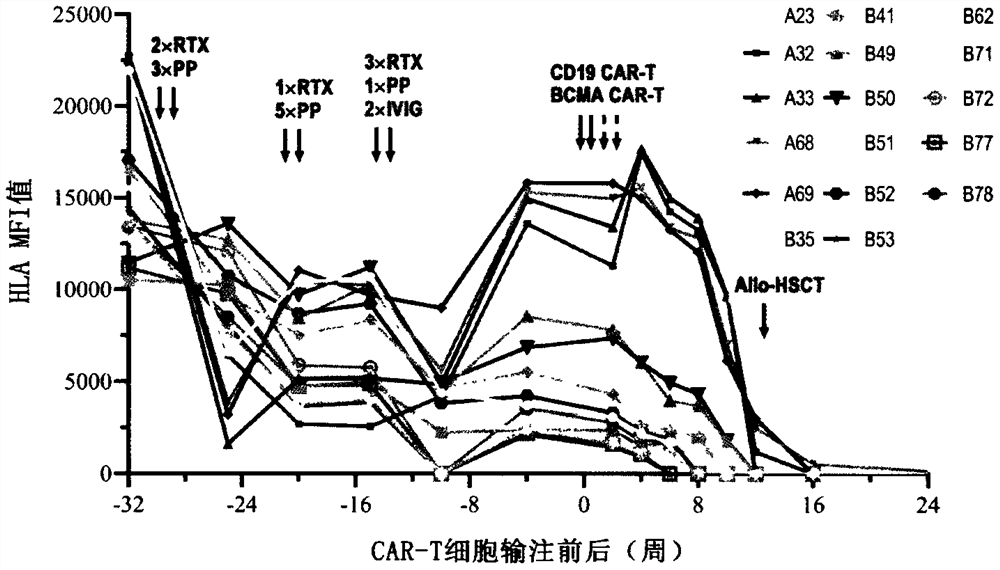
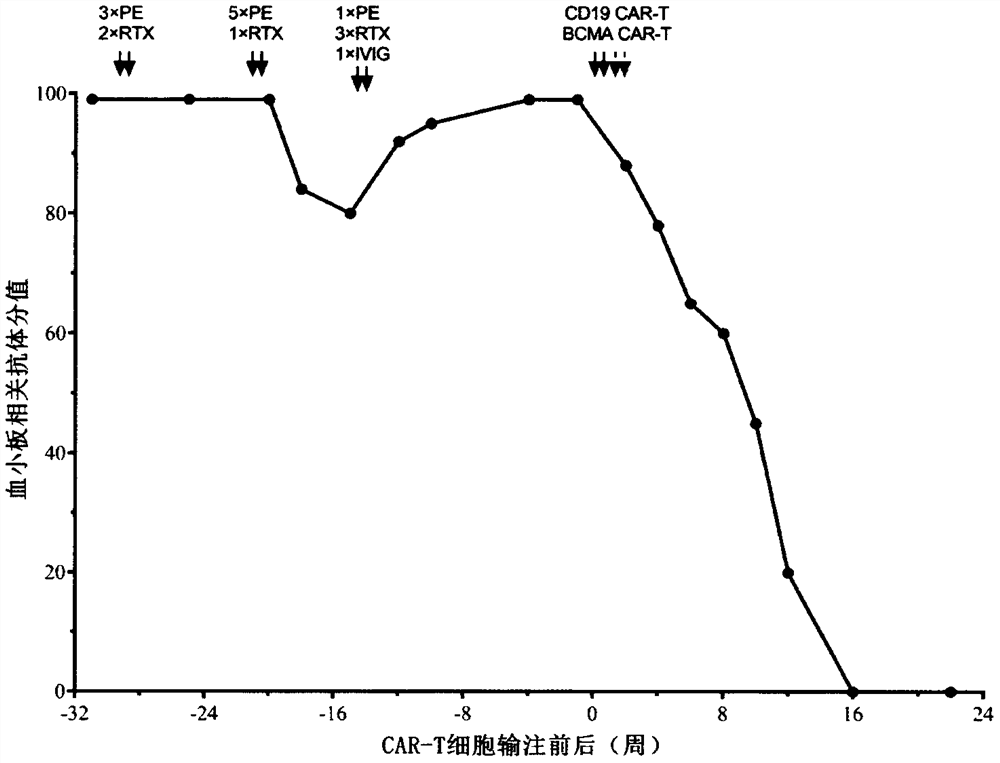
Combined application of sequential infusion CD19 CAR-T and BCMA CAR-T cells in immune-mediated platelet infusion invalidation of acute leukemia patients
An acute leukemia, combined application technology, applied in the direction of medical preparations containing active ingredients, drug combinations, antibody medical ingredients, etc., to achieve the effect of improving the ineffectiveness of infusion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0032] The present invention will be further described below in conjunction with specific cases.
[0033] Case 1:
[0034] A 38-year-old female patient was diagnosed with acute myeloid leukemia (M2) and had a history of pregnancy. She visited the doctor due to fever in September 2019. The blood routine showed: WBC 5.97×10 9 / L,NE 3.62×10 9 / L, HB 69g / L, PLT 13×10 9 / L, transferred to the local hospital for blood transfusion and other comprehensive treatment, 09-16 complete bone wear, morphology: high level of active bone marrow hyperplasia, 20.5% of protocells, AML-M2 not excluded; white immunity: analysis of 26.6% of immature The cell population was expressed for myeloid antigens, and 57.5% of myeloid differentiation antigens were abnormally expressed, which was in line with the AML-M2 phenotype, so he went to our hospital. Check peripheral blood original young 22%. Chromosome: 46XX, t(8;21)(q22;q22)[7] / 45, idem, -X[3]. Bone marrow multiplex PCR: AML1-ETO fusion gene tra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com