Fusion protein and its prepared drug and coding gene and msc containing the gene
A fusion protein and gene technology, applied in the field of genetic engineering, can solve problems such as poor therapeutic effect, achieve the effect of increasing the cell ratio and reducing the incidence of aGVHD
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] The connection of embodiment 1 target gene and carrier
[0021] 1. Design primers
[0022] Upstream primer: 5'-CGGAATTCGGATCCAGGCCTAAGCT-3'; (SEQ ID NO.5)
[0023] Downstream primer: 5'-CGGGATCCGAATTCGAAGTTGAGCTC-3'; (SEQ ID NO.6)
[0024] 2. Target gene PCR, the reaction system is: 1 μL DNA template (IFNG-IFNGR1, as shown in SEQ ID NO.4), 2 μL Primer-F (10 μM), 2 μL Primer-R (10 μM), 10 μL 2×MasterMix, Finally with ddH 2 O supplemented to 50 μL;
[0025] The reaction program was: preheating at 94°C for 3 min, denaturation at 94°C for 30 s, annealing at 55°C for 30 s, extension at 72°C for 1 min, a total of 25 cycles, and finally extension at 72°C for 10 min.
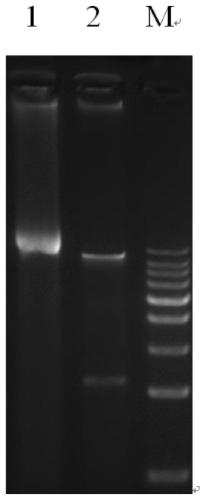
[0026] 3. Respectively digest the target gene and the vector, wherein the enzyme digestion system of the target gene includes: 25 μL PCR product, 5 μL Buffer EcoRI, 2.5 μL EcoRI, 2.5 μL BamHI, and finally use ddH 2 O supplemented to 50 μL;
[0027] The vector digestion system includes: 20 μL pWPXLD, 5 μL Bu...
Embodiment 2
[0031] Example 2 Lentiviral Packaging of Recombinant Plasmid pWPXLd-IFNG-IFNGR1
[0032] 1. Use the TIANGEN endotoxin-free plasmid extraction kit to extract the recombinant plasmid. The specific process is as follows:
[0033] 1. Put the adsorption column CP6 into a 50mL collection tube first, then add 2.5mL of balance solution BL, centrifuge at 8000r / min at room temperature for 2 minutes, discard the waste liquid in the collection tube, and put the adsorption column back into the collection tube;
[0034] 2. Take 100mL of overnight cultured bacterial solution into a centrifuge tube, centrifuge at room temperature at 8000r / min for 3 minutes to collect the bacteria, try to absorb the supernatant, and use clean absorbent paper to absorb the water droplets on the bottle wall;
[0035] 3. Add 8 mL of RNase A-containing solution P1 to the centrifuge tube with bacterial sediment, and vortex to completely suspend the bacterial sediment;
[0036] 4. Add 8 mL of solution P2 to the cen...
Embodiment 3
[0077] Example 3 Infection of human mesenchymal stem cells
[0078] 1. The mesenchymal stem cells were divided into 1.0×10 5 Inoculate 10% FBS, 100U / L double-antibody DMEM medium 2mL / well in 5% CO 2 , 37 ℃ cell incubator culture;
[0079] 2. When the inoculated mesenchymal stem cells adhere to the wall evenly and the confluence reaches 30%, calculate the required virus volume according to the measured virus titer according to the Moi value of 50;
[0080] 3. Add the required virus volume to 2 mL of DMEM medium with 10% FBS and 100 U / L double antibody;
[0081] 4. Suck off the original medium of mesenchymal stem cells in the 6-well plate, add 2 mL of virus-containing medium to each well; in 5% CO 2 , 37 ℃ cell incubator culture, need to observe the state of the cells during the culture;
[0082] 5. Change the medium after culturing for 12-16 hours;
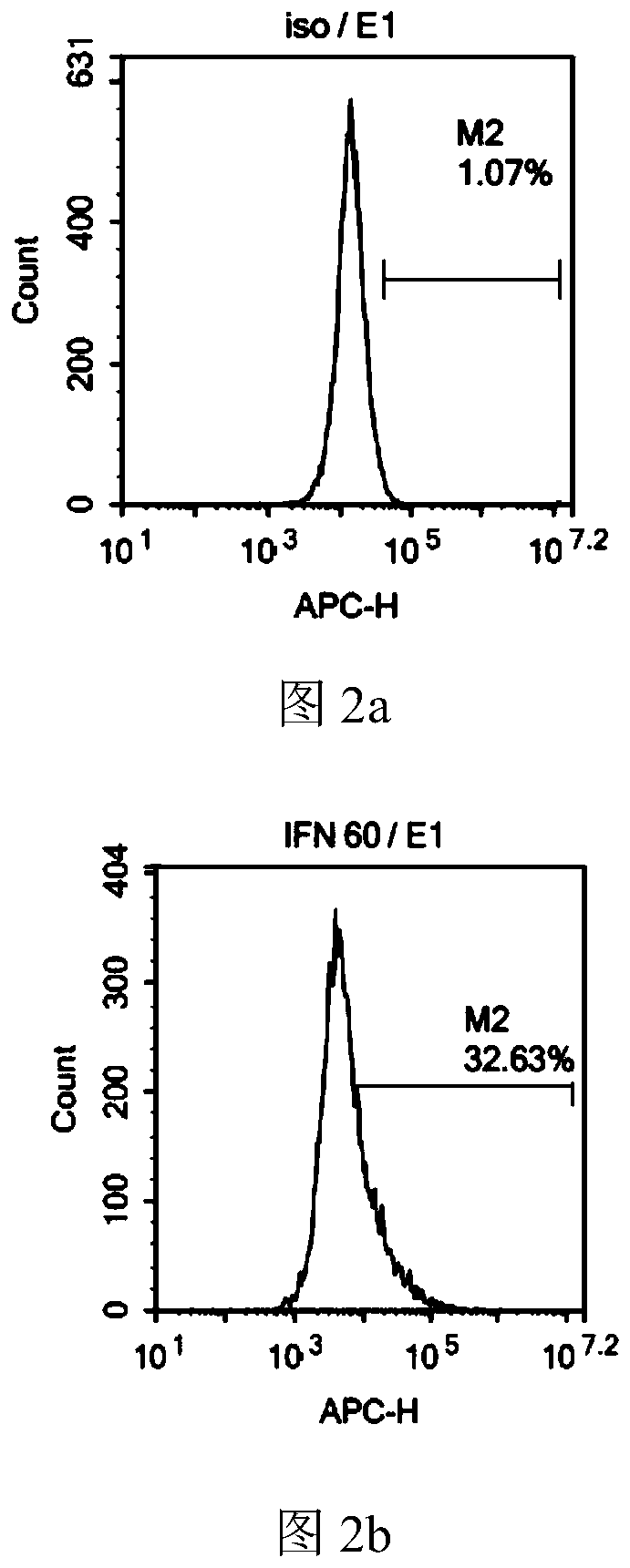
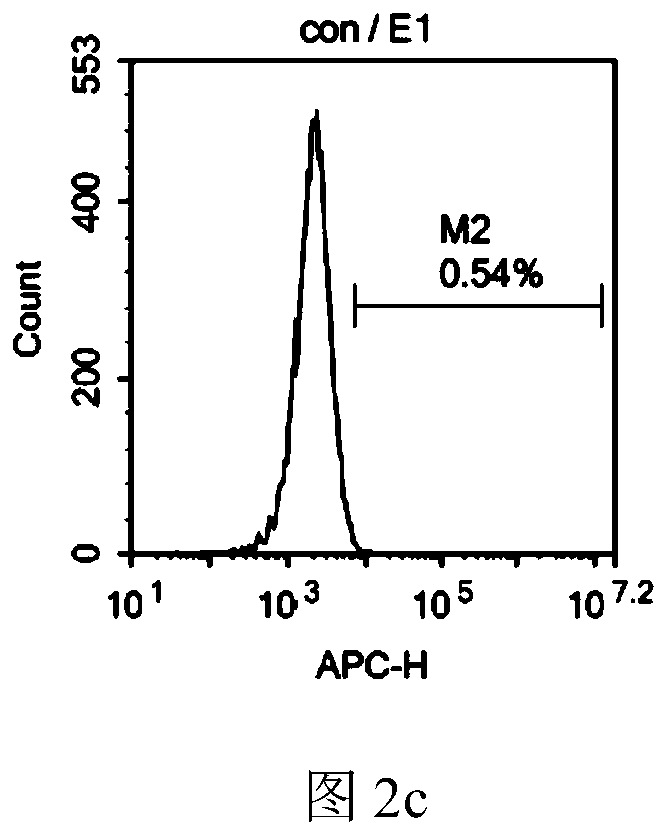
[0083] 6. After 72 hours of culture, the cells were harvested, the expression of Ifn-γ and Ifn-γR1 in the membrane was detec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com