Method for generating dumbbell-shaped DNA vector
A dumbbell-shaped, vector-based technology, applied in the direction of recombinant DNA technology, biochemical equipment and methods, microbial determination/inspection, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
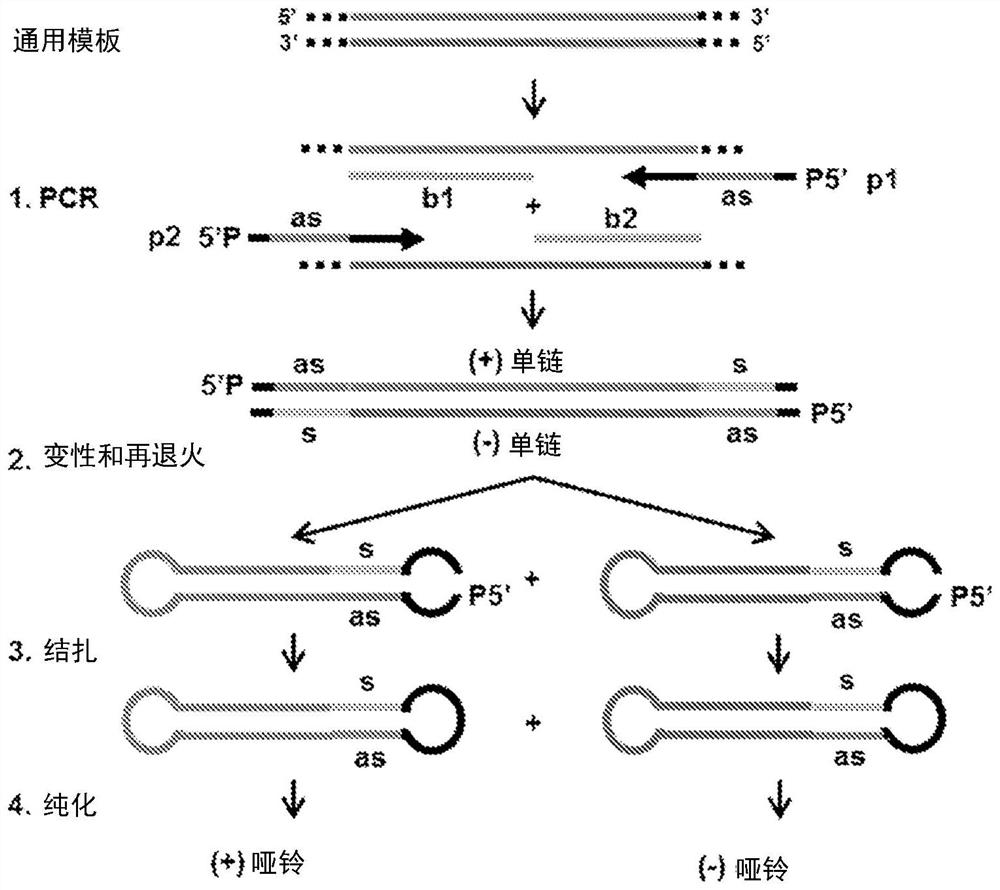
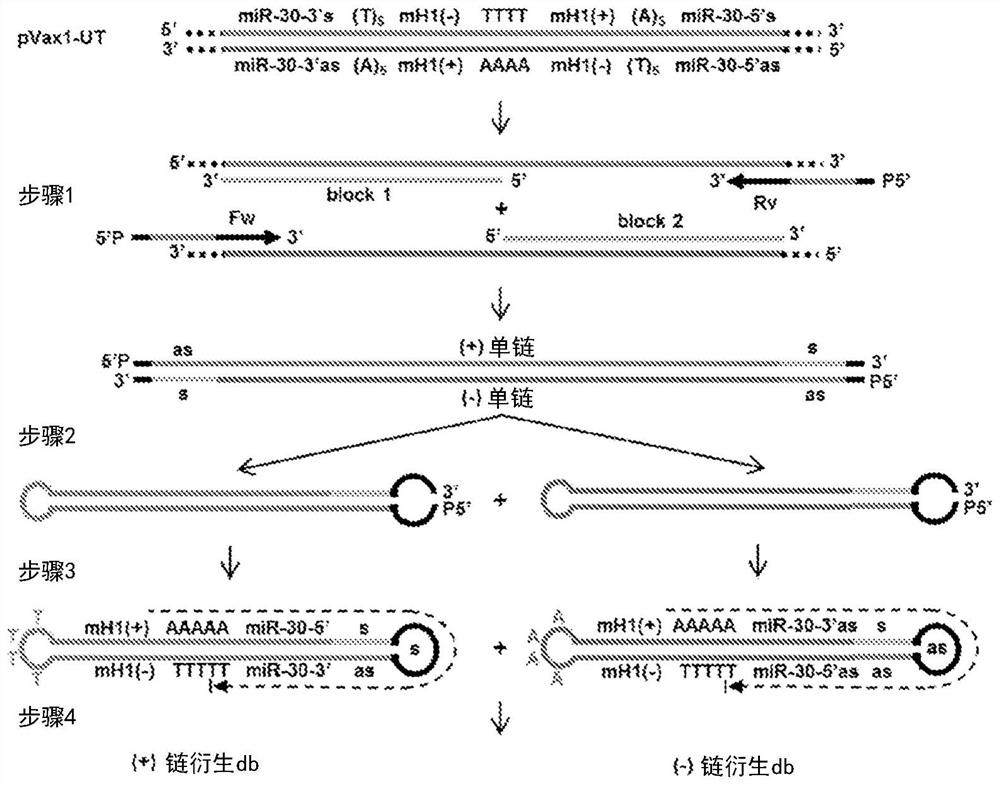
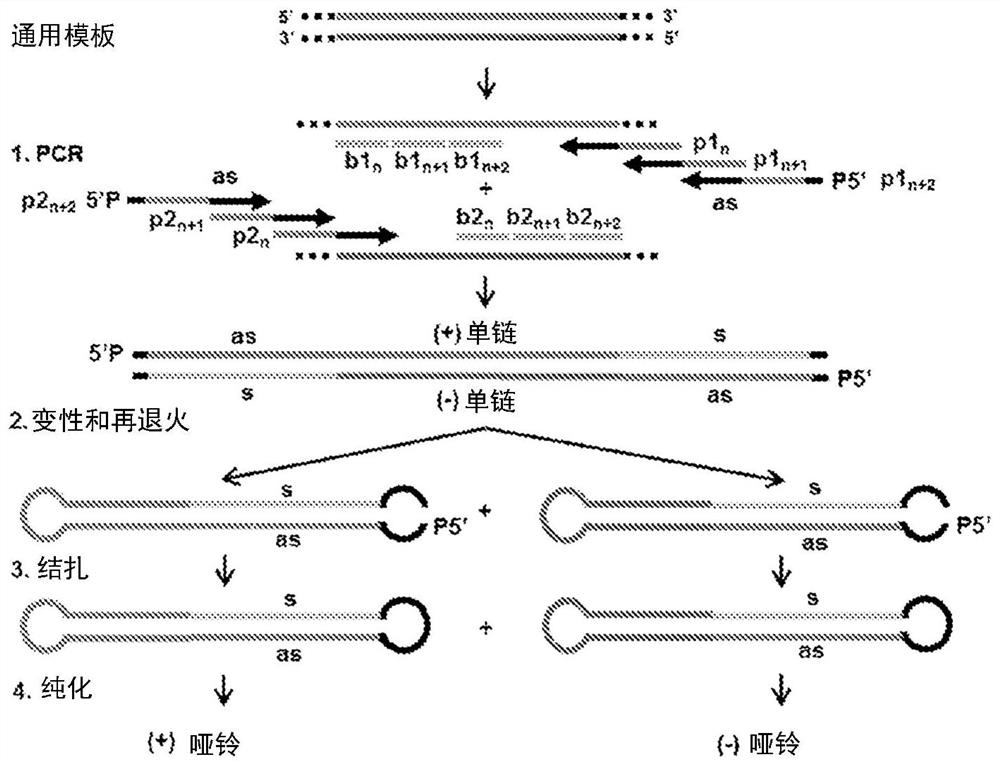
[0328]In the current state-of-the-art protocol, the generation of each new dumbbell vector begins with the individual cloning of the sequence of interest to be implemented into the dumbbell into a plasmid vector. The method of the present invention only needs to prepare a general template, including promoters, enhancers, DNA nuclear localization signals, introns, transcription terminators, RNA nuclear export signals, WPRE and other regulatory sequences. The sequence of interest is then introduced via chemically synthesized PCR primers without further cloning and without the need for endonucleases ( Figure 1-7 ). This generated dumbbell can be used as a molecular bait or an expression vector. Expression vectors can express noncoding RNAs, including shRNA, pre-miRNA, miRNA, aptamers, antisense RNA, and antisense miRNA ( Figure 1-4 , 6, 7) and / or expression of peptide or protein-coding RNA ( Figure 5-7 ). The expression cassettes of hairpin RNAs (such as shRNA and pre-miRN...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com