Quality detection method of lobedfruit schizocapsa herb medicinal material
A quality inspection method, the technology of seven medicinal materials, applied in the field of quality inspection of the seven medicinal materials of the paddy field, can solve the problems of the inability to effectively control the overall quality of the seven medicinal materials of the paddy fields, the inability to distinguish the seven medicinal materials of the paddy fields, and the lack of content determination items, etc., to achieve assurance Safety effectiveness, improved safety and stability, and high accuracy effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
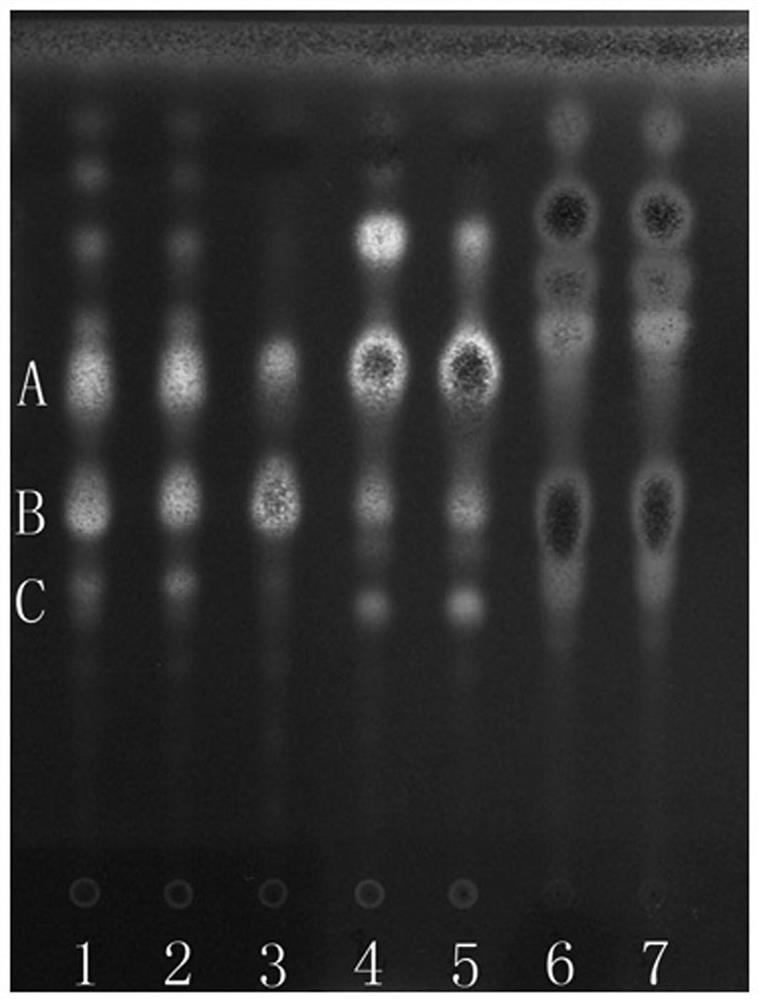
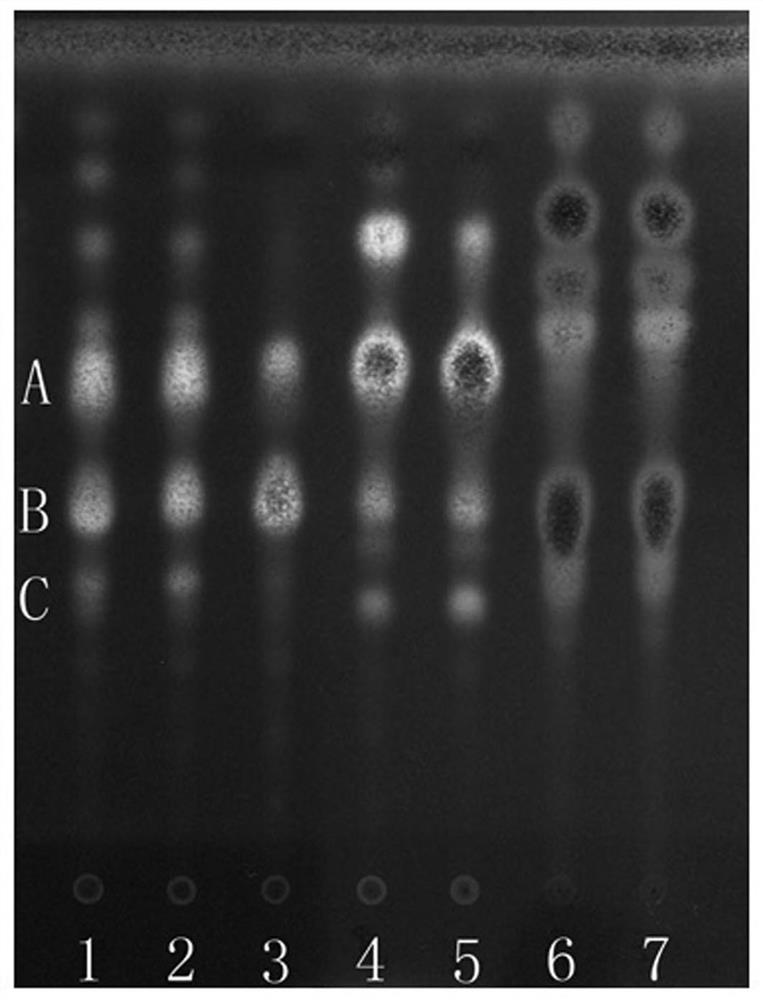
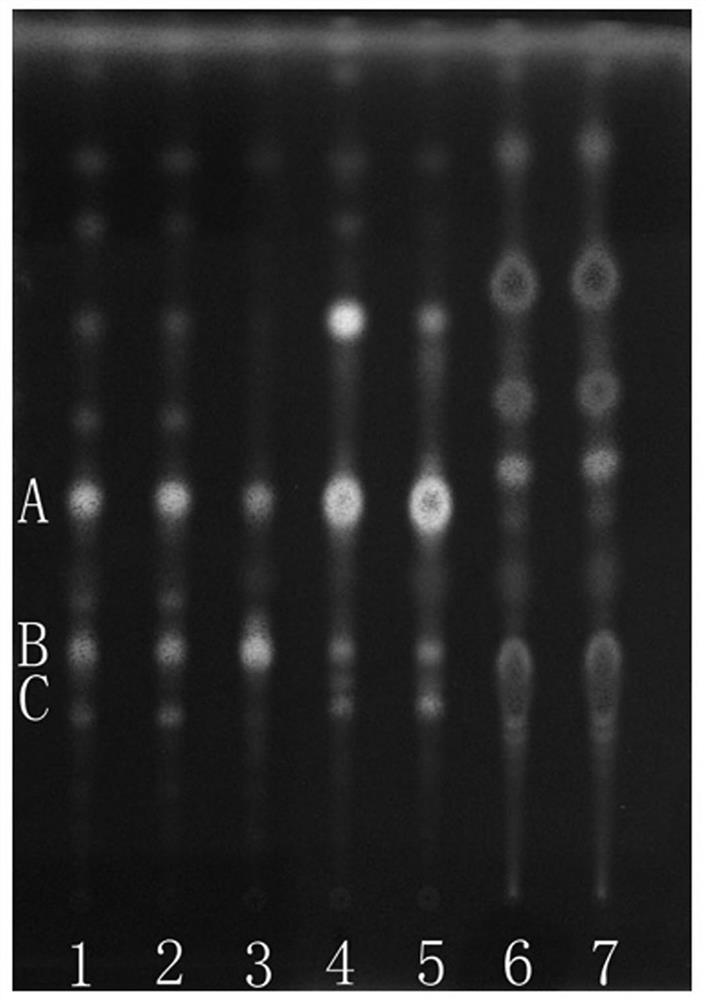
[0054] 1. Thin Layer Chromatography
[0055] 1 Test material
[0056] 1.1 Materials and reagents Prefabricated silica gel G thin-layer plates were purchased from Qingdao Ocean Chemical Co., Ltd., Yantai Chemical Industry Research Institute and Merck Company; other reagents were of analytical grade.
[0057] 1.2 The reference material, the reference medicinal material of Shuitianqi, was provided by the Guangxi Institute for Food and Drug Control; the reference medicinal material of arrowroot potato, batch number 121400-200401, was purchased from the China Institute for Food and Drug Control.
[0058] 1.3 Test article
[0059] 20 batches of paddy field seven samples, 4 batches of arrowroot potato and 5 batches of Panax notoginseng were collected or collected in the field, see Table 1 for details.
[0060]
[0061] 2 Test records
[0062] 2.1 Preparation of the test solution Take 1 g of P. chinensis sample powder, add 30 ml of methanol, ultrasonically treat it for 30 minute...
Embodiment 2
[0155] A quality detection method for the medicinal materials of Shuitianqi, including a method for identifying the medicinal materials of S. step:
[0156] (1) Take 1g of this product powder, add 30ml of methanol, sonicate for 30 minutes, filter, evaporate the filtrate to dryness, add 30ml of water to the residue to dissolve, shake and extract with ethyl acetate twice, 30ml each time, discard the ethyl acetate Then, shake and extract 2 times with water-saturated n-butanol, 30 ml each time, combine the n-butanol solution, evaporate to dryness, add 1 ml of methanol to the residue to dissolve it, and use it as the test solution; method to prepare the reference medicinal material solution;
[0157] (2) According to the test of thin-layer chromatography (General Chapter 0502 of the Fourth Part of the Chinese Pharmacopoeia 2020), draw 1 μl of each of the above two solutions, and place them on the same silica gel G thin-layer plate respectively. 1:1) as developing agent, develop, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com