Preparation method of chiral alpha-hydroxy-beta-keto ester compound
A ketoester and compound technology, applied in the field of organic asymmetric catalysis, can solve the problems of expensive catalyst, large catalytic amount of catalyst, low enantioselectivity, etc., and achieve the effects of efficient asymmetric synthesis, low cost and simple synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
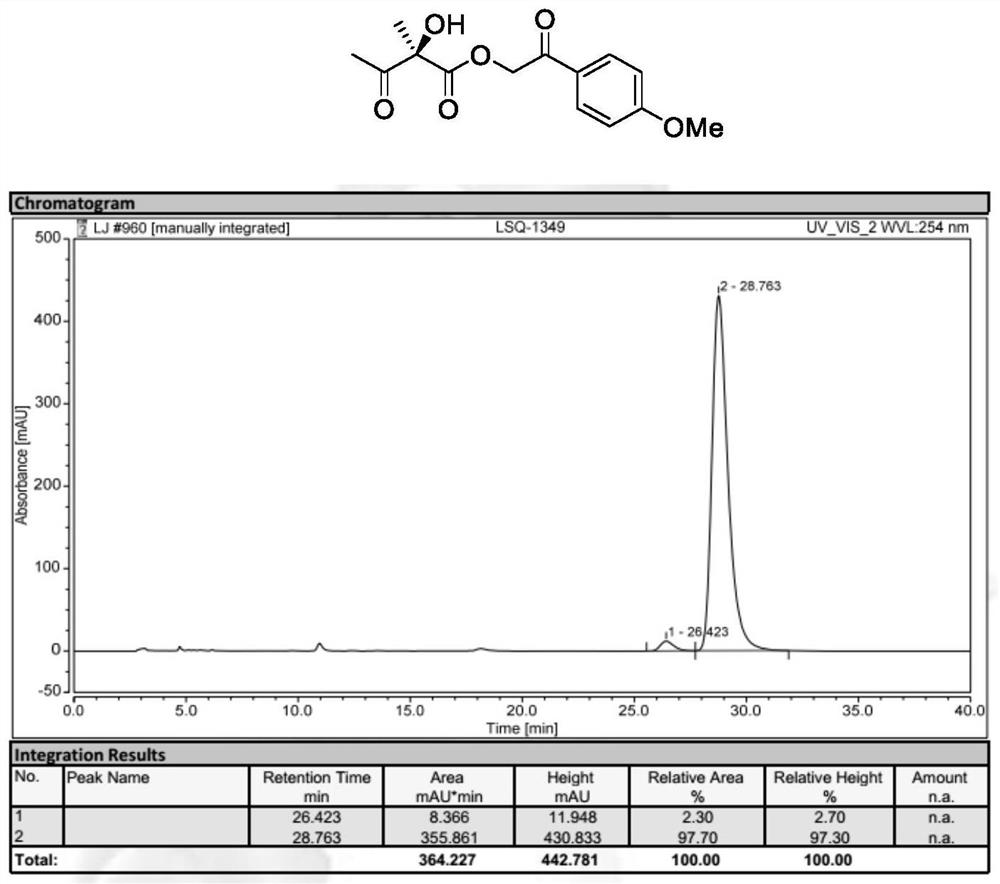
[0038] Preparation of 2-(4-Methoxyphenyl)-2-oxoethyl (S)-2-hydroxy-2-methyl-3-oxobutyrate (Formula II, wherein R 1 ,R 2 is methyl, R 3 For p-methoxyacetophenone group):
[0039]
[0040] 2-(4-Methoxyphenyl)-2-oxoethyl (E)-2-methylbut-2-enoic acid (formula I, wherein R 1,R 2 is methyl, R 3 For p-methoxyacetophenone) (49.6mg, 0.20mmol), N-3,5-difluorobenzyl-O-2-bromo-3,5-di-tert-butylbenzyl quinaline quaternary A mixture of ammonium salt phase transfer catalyst Cat.1 (7.8 mg, 5 mol%) in toluene (4 mL) was cooled to -20°C, and then acetic acid (60.0 mg, 5 eq.), potassium permanganate (63.2 mg) were added to it sequentially , 2eq.) and 40% aqueous KF. The mixture was reacted at -20°C for 12 hours. After the complete reaction of the starting materials, the reaction mixture was filtered. The solvent was then re-evaporated and purified rapidly with a silica gel column. 2-(4-Methoxyphenyl)-2-oxoethyl (S)-2-hydroxy-2-methyl-3-oxobutyrate gave 96% yield with enantiomeric Th...
Embodiment 2
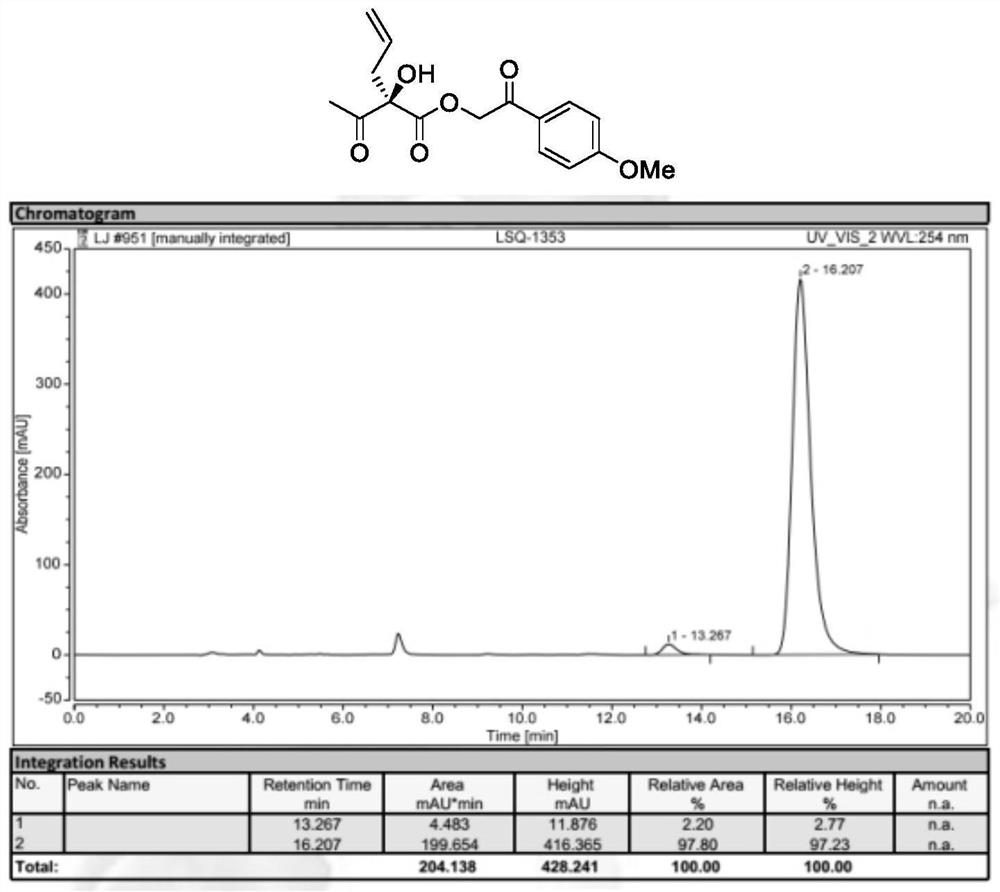
[0050] Preparation of 2-(4-Methoxyphenyl)-2-oxoethyl (S)-2-acetyl-2-hydroxypent-4-enoate (Formula II, wherein R 1 is allyl, R 2 is methyl, R 3 for p-methoxyacetophenone group)
[0051]
[0052] 2-(4-Methoxyphenyl)-2-oxoethyl (E)-2-ethylenepent-4-enoate (Formula I, where R 1 is allyl, R 2 is methyl, R 3 For p-methoxyacetophenone) (54.8mg, 0.20mmol), N-3,5-difluorobenzyl-O-2-bromo-3,5-di-tert-butylbenzyl quinaline quaternary A mixture of ammonium salt phase transfer catalyst Cat.1 (7.8 mg, 5 mol%) in TBME (4 mL) was cooled to -40°C, and then acetic acid (60.0 mg, 5 eq.), potassium permanganate (63.2 mg) were added to it sequentially , 2eq.) and 40% aqueous KF. The mixture was reacted at -40°C for 12 hours. After the complete reaction of the starting materials, the reaction mixture was filtered. The solvent was then re-evaporated and purified rapidly with a silica gel column. 2-(4-Methoxyphenyl)-2-oxoethyl (S)-2-acetyl-2-hydroxypent-4-enoate gave 89% yield with enanti...
Embodiment 3
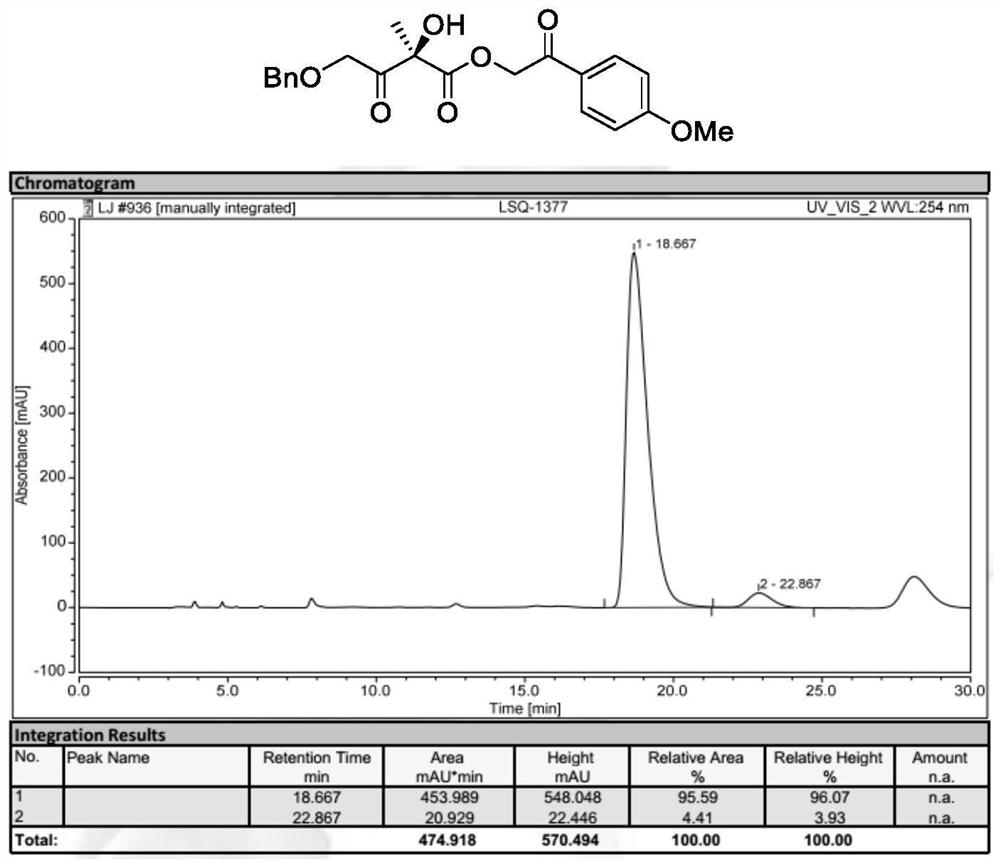
[0055] Preparation of 2-(4-Methoxyphenyl)-2-oxoethyl (S)-2-hydroxy-2-methyl-3-oxobutyrate (Formula II, wherein R 1 ,R 2 is methyl, R 3 For p-methoxyacetophenone group):
[0056]
[0057] 2-(4-Methoxyphenyl)-2-oxoethyl (S)-2-acetyl-2-hydroxypent-4-enoate (formula I, wherein R 1 is benzyl, R 2 is methyl, R 3 as p-methoxyacetophenone) (49.6 mg, 0.20 mmol), N-3,4-difluorobenzyl-O-2-bromo-3,5-bis(3,5-di-tert-butyl) A mixture of phenylbenzyl bromide cinchona-based quaternary ammonium salt phase transfer catalyst Cat.2 (9.7 mg, 5 mol%) in TBME (4 mL) was cooled to 0°C, and then acetic acid (60.0 mg, 5 eq.) was sequentially added thereto. , potassium permanganate (63.2 mg, 2 eq.) and 200 microliters of water. The mixture was reacted at 0°C for 12 hours. After the complete reaction of the starting materials, the reaction mixture was filtered. The solvent was then re-evaporated and purified rapidly with a silica gel column. 2-(4-Methoxyphenyl)-2-oxoethyl (S)-2-hydroxy-2-methyl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com