Preparation method of chiral alpha-hydroxy-beta-keto ester compound
A technology of keto esters and compounds, which is applied in the field of organic asymmetric catalysis, can solve the problems of expensive catalysts, large catalytic capacity, and low enantioselectivity, and achieve high-efficiency asymmetric synthesis, low cost, and simple synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
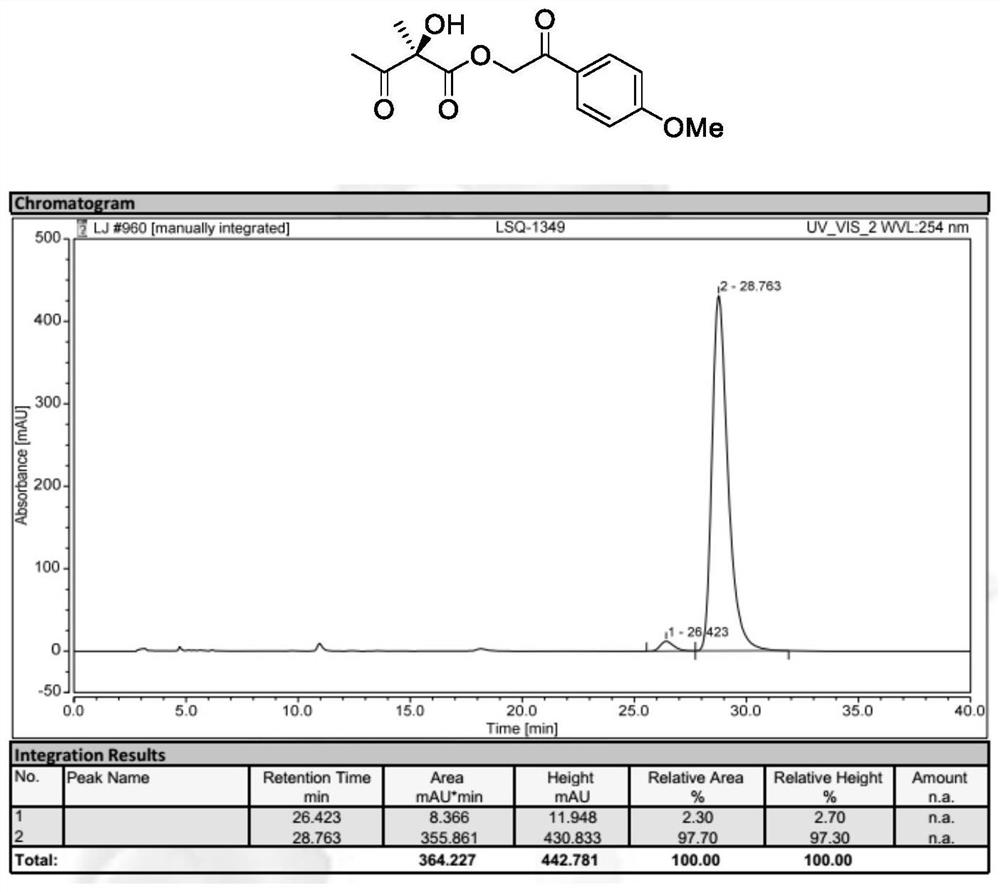
[0038] Preparation of 2-(4-methoxyphenyl)-2-oxoethyl (R)-2-hydroxyl-2-methyl-3-oxobutyrate (formula Ⅱ, wherein R 1 , R 2 is methyl, R 3 is p-methoxyacetophenone group):
[0039]
[0040] 2-(4-methoxyphenyl)-2-oxyethyl (E)-2-methylbut-2-enoic acid (formula I, where R 1 , R2 is methyl, R 3 It is p-methoxyacetophenonyl) (49.6mg, 0.20mmol), N-3,5-difluorobenzyl-O-2-bromo-3,5-di-tert-butylbenzyl quinone base quaternary The mixture of ammonium salt phase transfer catalyst Cat.1 (7.8mg, 5mol%) in toluene (4mL) was cooled to -20°C, then acetic acid (60.0mg, 5eq.), potassium permanganate (63.2mg , 2eq.) and 40% KF aqueous solution. The mixture was reacted at -20°C for 12 hours. After the starting material was completely reacted, the reaction mixture was filtered. The solvent was then evaporated again and flash purified on a silica gel column. 2-(4-Methoxyphenyl)-2-oxoethyl(R)-2-hydroxy-2-methyl-3-oxobutanoate was obtained in 96% yield and enantiomeric The ee value is 95%. ...
Embodiment 2
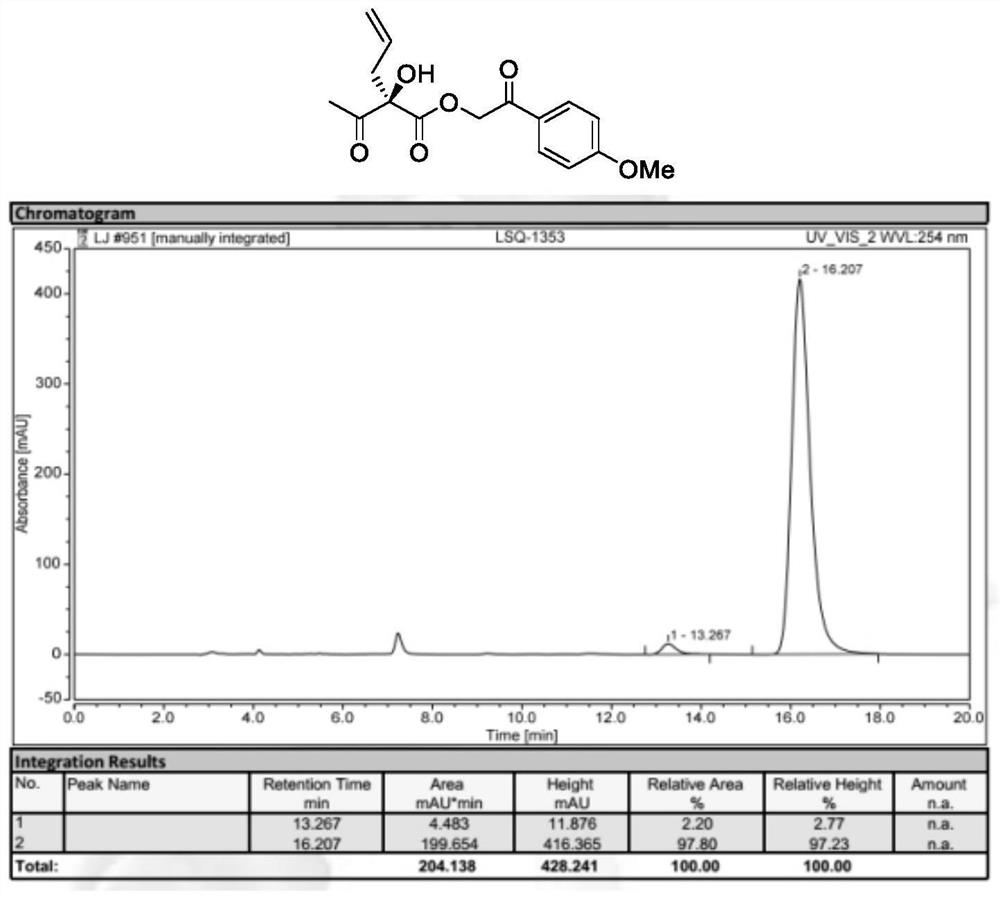
[0043] Preparation of 2-(4-methoxyphenyl)-2-oxoethyl (R)-2-acetyl-2-hydroxypent-4-enoate (formula II, where R 1 is allyl, R 2 is methyl, R 3 for p-methoxyacetophenone)
[0044]
[0045] 2-(4-methoxyphenyl)-2-oxyethyl (E)-2-ethylenepenta-4-enoate (formula I, where R 1 is allyl, R 2 is methyl, R 3 It is p-methoxyacetophenonyl) (54.8mg, 0.20mmol), N-3,5-difluorobenzyl-O-2-bromo-3,5-di-tert-butylbenzyl quinone base quaternary The mixture of ammonium salt phase transfer catalyst Cat.1 (7.8mg, 5mol%) in TBME (4mL) was cooled to -40°C, then acetic acid (60.0mg, 5eq.), potassium permanganate (63.2mg , 2eq.) and 40% KF aqueous solution. The mixture was reacted at -40°C for 12 hours. After the starting material was completely reacted, the reaction mixture was filtered. The solvent was then evaporated again and flash purified on a silica gel column. 2-(4-Methoxyphenyl)-2-oxoethyl(R)-2-acetyl-2-hydroxypent-4-enoate was obtained in 89% yield, and the enantiomeric ee The value ...
Embodiment 3
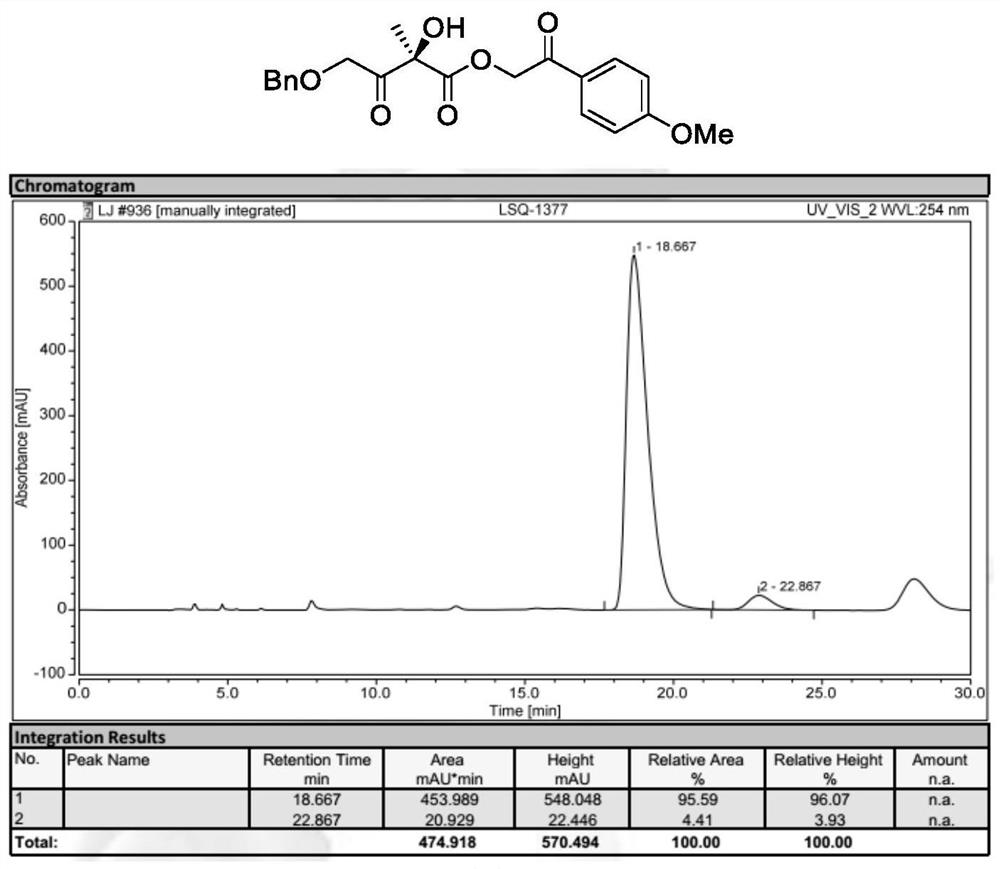
[0048] Preparation of 2-(4-methoxyphenyl)-2-oxoethyl (R)-2-hydroxyl-2-methyl-3-oxobutyrate (formula Ⅱ, wherein R 1 , R 2 is methyl, R 3 is p-methoxyacetophenone group):
[0049]
[0050] 2-(4-methoxyphenyl)-2-oxoethyl (R)-2-acetyl-2-hydroxypent-4-enoate (formula I, where R 1 is benzyl, R 2 is methyl, R 3 p-methoxyacetophenonyl) (49.6mg, 0.20mmol), N-3,4-difluorobenzyl-O-2-bromo-3,5-di(3,5-di-tert-butyl) The mixture of phenylbenzylcinchona base quaternary ammonium phase transfer catalyst Cat.2 (9.7mg, 5mol%) in TBME (4mL) was cooled to 0°C, and then acetic acid (60.0mg, 5eq.) was added successively thereto , potassium permanganate (63.2 mg, 2 eq.) and 200 microliters of water. The mixture was reacted at 0°C for 12 hours. After the starting material was completely reacted, the reaction mixture was filtered. The solvent was then evaporated again and flash purified on a silica gel column. 2-(4-Methoxyphenyl)-2-oxoethyl(R)-2-hydroxy-2-methyl-3-oxobutanoate was obtained ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com