Triazolopyrimidine derivative as well as preparation method and application thereof
A technology for pyrimidine and derivatives, which is applied in the field of triazolopyrimidine derivatives and their preparation, and can solve problems such as research on USP28 inhibitors in the clinical stage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
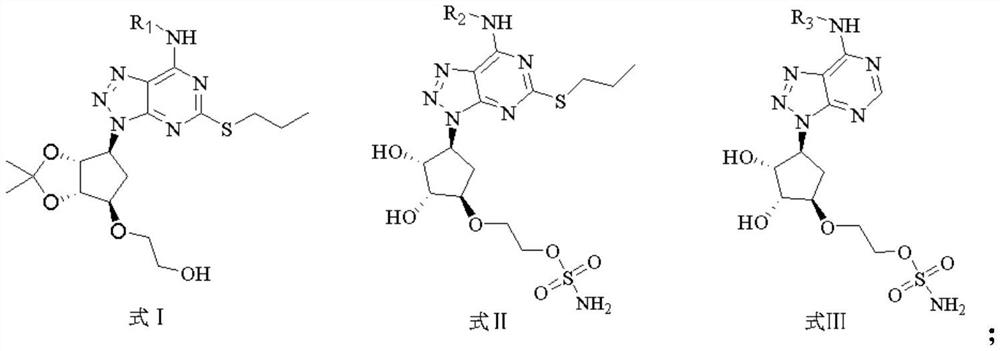
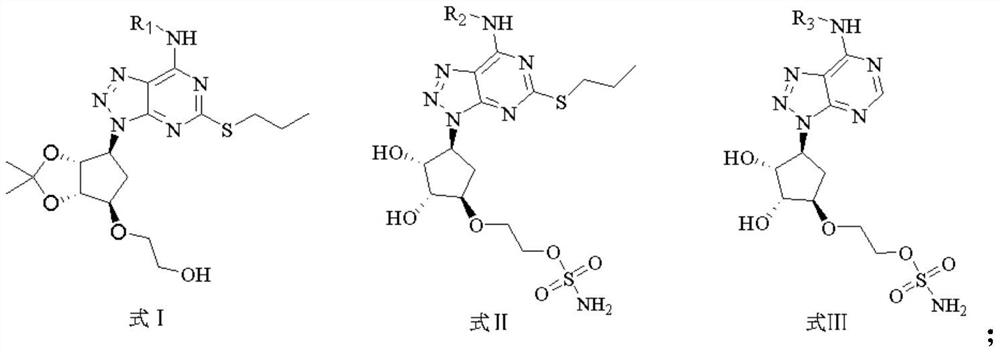
[0036] The triazolopyrimidine derivatives of this embodiment are compounds of the general formula I, in the general formula I, R 1 is cyclopropyl. This triazolopyrimidine derivative is referred to as compound 1. The preparation method of compound 1 is as follows:
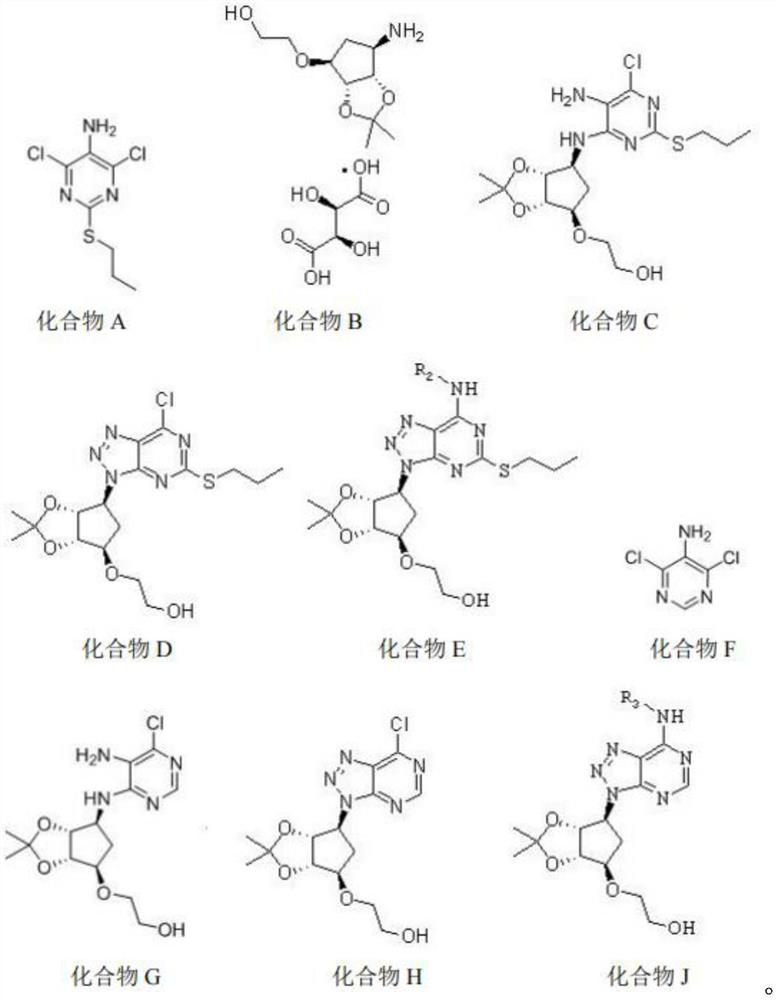
[0037] 1) Synthesis of compound C, compound A (50.0 g, 1.02 eq.) and compound B (75.6 g, 1.0 eq.) were placed in a 5L autoclave, and 750 mL of ethanol was added to form a suspension. Under stirring, triethylamine (62.50 g, 3.0 eq.) was added to the suspension, heated to 125° C., and kept stirring for 30 h. After cooling to room temperature, the reaction was monitored by TLC (PE:EA=1:1). Most of the solvent was distilled off under reduced pressure, ethyl acetate and water were added, and 6M hydrochloric acid solution was added under stirring to adjust the pH of the aqueous phase to 5. After standing to separate layers, the organic phase was washed three times with water and once with saturated brine. The organic...
Embodiment 2~38
[0041] The triazolopyrimidine derivatives of Examples 2 to 38 all have the general formula I, R 1 Selected in order from cyclohexyl, 4-methylpiperidyl, 4-methylpiperazinyl, n-propyl, 2-hydroxyethyl, 2-methoxyethyl, 2-aminoethyl, 2-(acetyl amino)ethyl, 2-(phenylamino)ethyl, 2-(benzoate amino)ethyl, 2-(tert-butoxycarbonylamino)ethyl, 2-(dimethylamino)ethyl base, 2-(tert-butoxycarbonylmethylamino)ethyl, (2-(4-phenylpiperazin-1-yl)ethyl, benzyl, 4-pyridyl, 2-fluorobenzyl, 2- chlorobenzyl, 2-bromobenzyl, 2-methylbenzyl, 2-methoxybenzyl, 3-fluorobenzyl, 3-chlorobenzyl, 3-bromobenzyl, 3-methylbenzyl, 3-methoxybenzyl, 4-fluorobenzyl, 4-chlorobenzyl, 4-bromobenzyl, 4-methylbenzyl, 4-methoxybenzyl, 4-trifluoromethylbenzyl, 3,5-dimethoxybenzyl, 4-methoxyphenyl, 4-chlorophenyl, 2,3-dihydro-1H-inden-1-yl, benzo[d][1,3] Dioxolane-4-yl, the obtained triazolopyrimidine derivatives are sequentially denoted as compounds 2 to 38. The preparation methods of compounds 2 to 38 are basically the ...
Embodiment 39
[0080] The triazolopyrimidine derivatives of this embodiment are compounds of the general formula II, in the general formula II, R 2 is cyclopropyl. This triazolopyrimidine derivative is designated as compound 39. The specific preparation method of compound 39 is as follows: wherein, step 1) synthesis of compound C, step 2) synthesis of compound D is the same as that of Example 1.
[0081] 3) Synthesis of compound E: put the intermediate compound D (200mg, 1.0eq.) in a 25mL thick-walled eggplant-shaped flask, dissolve in anhydrous acetonitrile, and add N,N-diisopropylethylamine (92mg, 1.5 eq.). Under the ice bath, amine substance - cyclopropylamine (40mg, 1.5eq.) was added dropwise. The mixture was stirred at room temperature for 8 h, and after the reaction was monitored by TLC (PE:EA=2:1), the solvent was distilled off under reduced pressure. It was extracted three times with ethyl acetate and washed once with water. The organic phase was dried over anhydrous sodium sulf...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com