Chemical component limited culture medium for HEK293 cell culture and replication and amplification of adenovirus and adeno-associated virus
A cell culture and chemical composition technology, applied in the direction of animal cells, vertebrate cells, artificial cell constructs, etc., can solve the harsh transportation and storage conditions of culture medium products, inconvenient transportation and storage, inconvenient solution for customers, and insufficient product titer, etc. problems, to achieve the effect of ensuring cell viability and expansion speed, high product safety and stability, and loose transportation and storage conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] The components of the chemical composition-limited culture medium of the present embodiment and their contents are as follows:
[0073] The composition and content of amino acids are:
[0074]
[0075] The components of the vitamin and its content are:
[0076]
[0077] The composition and content of trace elements are:
[0078]
[0079]
[0080] The components and contents of inorganic salts are:
[0081]
[0082] The composition and content of fatty acids are:
[0083] Linoleic acid 0.5mg / L
[0084] Linolenic acid 0.3mg / L.
[0085] Prepare the raw material components according to the above specific contents, fully dissolve them in sterile deionized water, adjust the pH to 6.9-7.0, adjust the volume to 10L, filter with a filter membrane with a pore size of 0.22 μm, and store at 4°C for later use (hereinafter referred to as CDM medium).
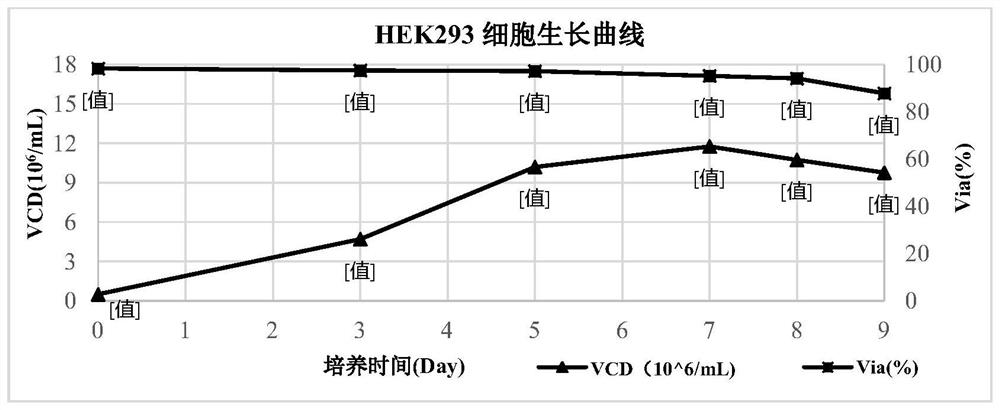
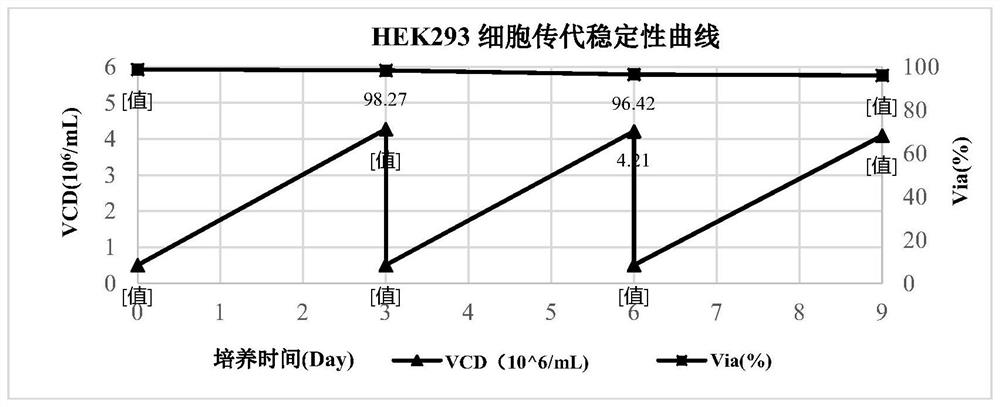
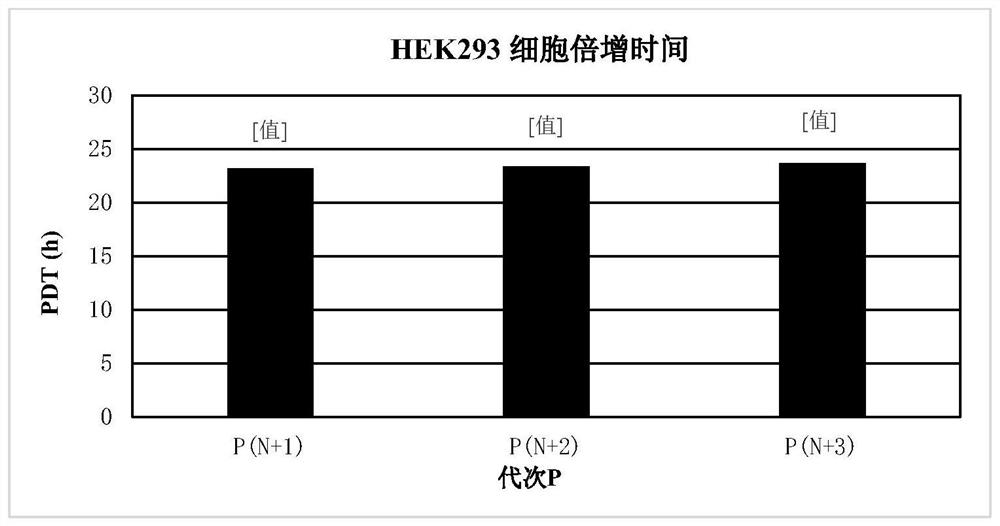
[0086] The cells used in this example were HEK293 cells, which were purchased from CTCC (Mason cells) in China. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Titer | aaaaa | aaaaa |
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com