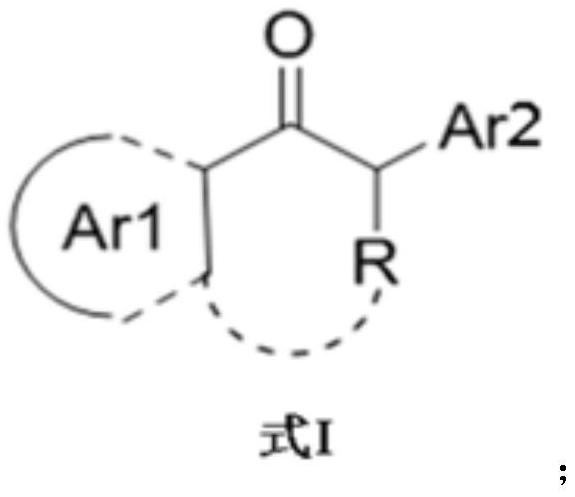
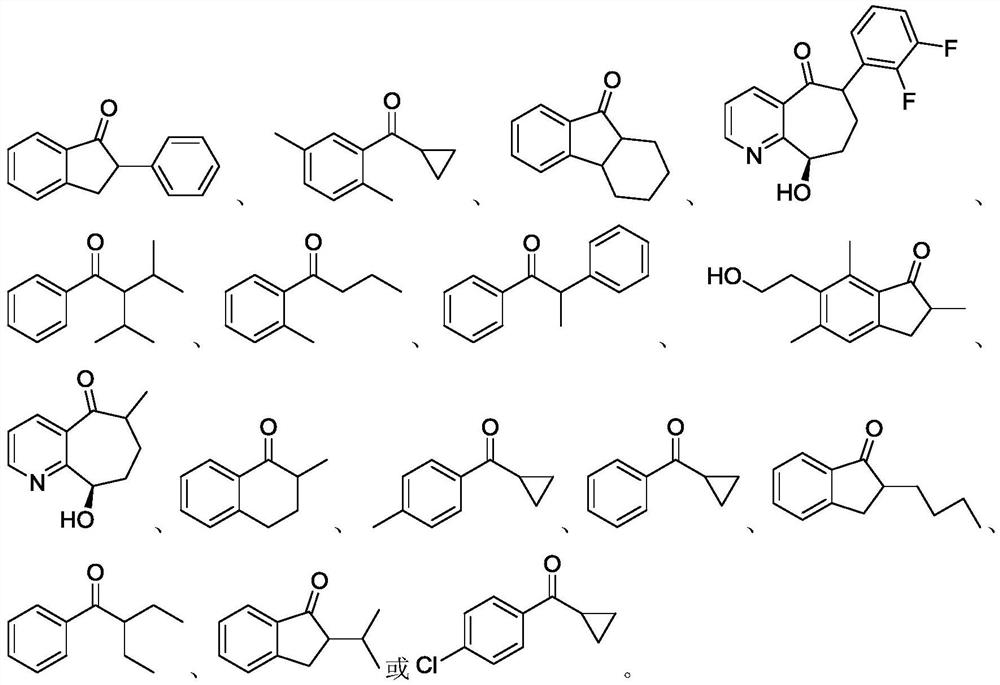
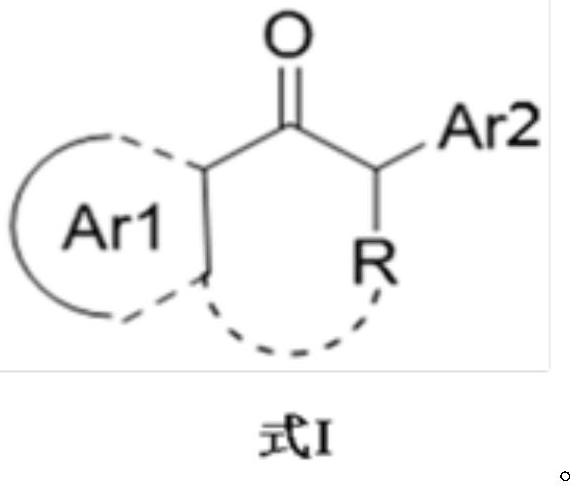
Preparation method of transaminase mutant and chiral amine compound
A transaminase and mutant technology, applied in the field of enzyme catalysis, can solve the problem of low transaminase activity, and achieve the effects of high enzyme activity, wide substrate spectrum, and strong ability to withstand extreme environments
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0233] Using the method described above, site-directed mutagenesis was performed on the basis of the parental SEQ ID NO:1. The specific mutation sites are shown in Table 1, and the catalytic activity of the mutants was detected according to the following reaction conditions:
[0234] 1mL reaction system includes 2mg substrate 1 or substrate 2 or substrate 3 or substrate 4, 1mg PLP, 2mg isopropylamine hydrochloride, 400μL crude enzyme solution (prepared by 200mg wet bacteria mud), pH8.0 100mM phosphoric acid Salt buffer, react at 50°C for 42h. The above-mentioned wet bacteria mud is obtained by centrifuging the corresponding mutant Escherichia coli fermentation broth, and the crude enzyme liquid is obtained by adding pH8.0100mM phosphate buffer to the obtained wet bacteria mud, homogenizing the walls by ultrasonic or homogenizer, and then concentrating.
[0235] The test results are shown in Table 1.
[0236] Table 1:
[0237]
[0238] Note: In the above table, 0 means th...
Embodiment 2
[0240] On the basis of Example 1, the combined mutation was performed, and the activity of the combined mutation was screened according to the same reaction conditions as Example 1. The results are shown in Table 2.
[0241] Table 2:
[0242]
[0243] Note: In the above table, 0 means the conversion rate is less than 1%, + means the conversion rate is greater than or equal to 1% and less than 5%, ++ means the conversion rate is greater than or equal to 5% and less than or equal to 10%, and +++ means the conversion rate is greater than or equal to 10% and less than 15%, ++++ means the conversion rate is greater than or equal to 15% and less than 20%.
Embodiment 3
[0245] On the basis of Example 2, multiple rounds of saturation mutation were carried out, and the catalytic activity of the mutant was detected according to the following reaction conditions:
[0246] 1mL reaction system includes 4mg substrate 1 or substrate 2 or substrate 3 or substrate 4, 1mg PLP, 2mg isopropylamine hydrochloride, 400μL crude enzyme solution (prepared by 200mg wet bacteria mud), pH8.0 100mM phosphoric acid Salt buffer, 50 ℃ reaction 18h. The results are shown in Table 3.
[0247] table 3:
[0248]
[0249]
[0250] Note: In the above table, 0 means the conversion rate is less than 1%, + means the conversion rate is greater than or equal to 1% and less than 5%, ++ means the conversion rate is greater than or equal to 5% and less than 10%, and +++ means the conversion rate is greater than or equal to 10 % and less than 15%, ++++ means the conversion rate is greater than or equal to 15% and less than 20%, +++++ means the conversion rate is greater than...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com