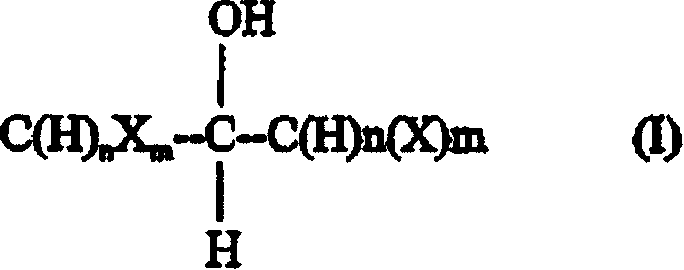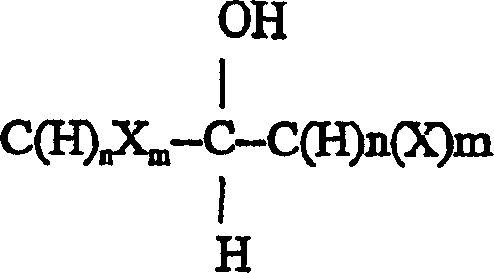Synthetic method for fluoromethylation of halogenated alcohols
A technology of fluoromethylation and halomethyl ether, which is applied in the field of fluoromethylation of haloalcohols, can solve problems such as affecting reversible regulation, complexity, and cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
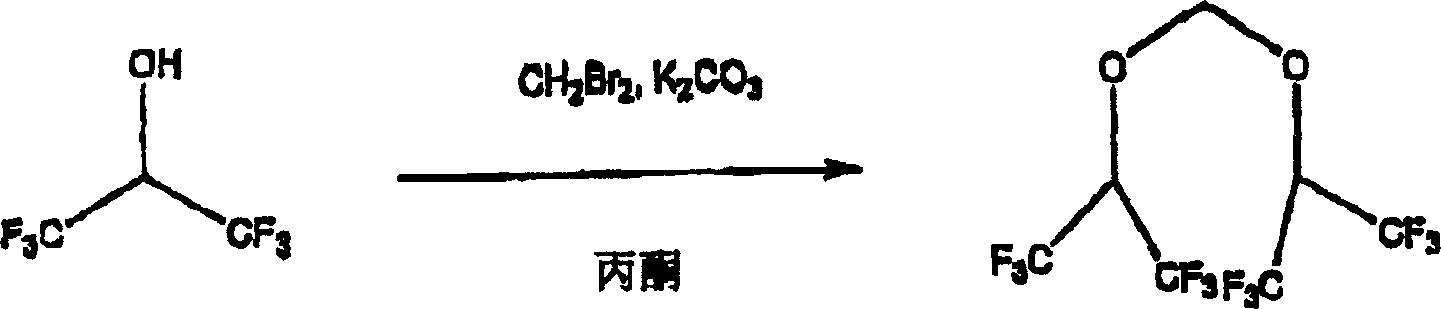
[0040] Bis(1,1,1,3,3,3-hexafluoroisopropoxy)methane was synthesized according to the following reaction scheme I:
[0041] To a solution of 1,1,1,3,3,3-hexafluoroisopropanol (1.5 mL, 15 mmol) and dibromomethane (1.6 mL, 23 mmol) in acetone (5.0 mL) was added K 2 CO 3 (3.15 gm, 23 mmol), the reaction was heated at reflux. After 18 hours the reaction mixture was cooled and filtered to remove solids. The filtrate was distilled to obtain bis(1,1,3,3,3-hexafluoroisopropoxy)methane (1.5 g, 52%). This stable acetal precursor is deprotected and fluorinated to sevoflurane by fluorination methods well known to those skilled in the art.
[0042]
[0043] 1,1,1,3,3,3-Hexafluoroisopropanol Bis(1,1,1,3,3,3-hexafluoroisopropoxy)methane
[0044] Reaction scheme I
Embodiment 2
[0046] Sevoflurane was synthesized according to the following reaction scheme II:
[0047] K 2 CO 3 (31.5g, 228mmol) and KF (17.5g, 300mmol), the reaction mixture was heated to 100°C. After 18 hours, analysis of the reaction mixture by gas chromatography (GC) showed 92% conversion of HFIP to sevoflurane. The reaction mixture was diluted with water (100 mL), the lower organic layer was separated and distilled to give sevoflurane (12 g, 40%).
[0048]
[0049] Reaction Scheme II
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com