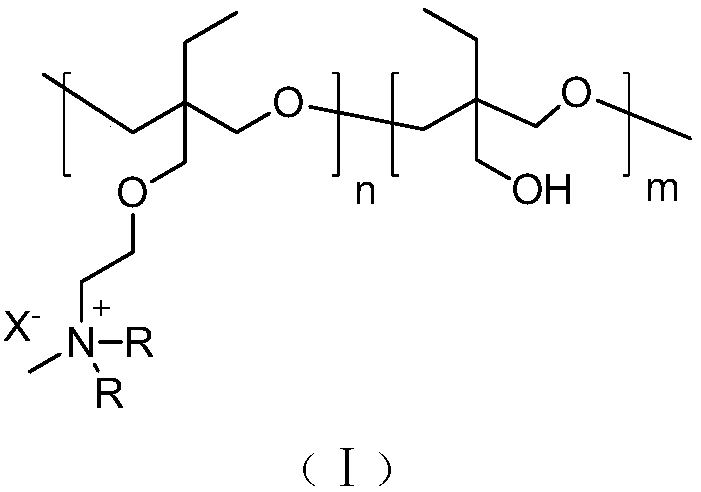
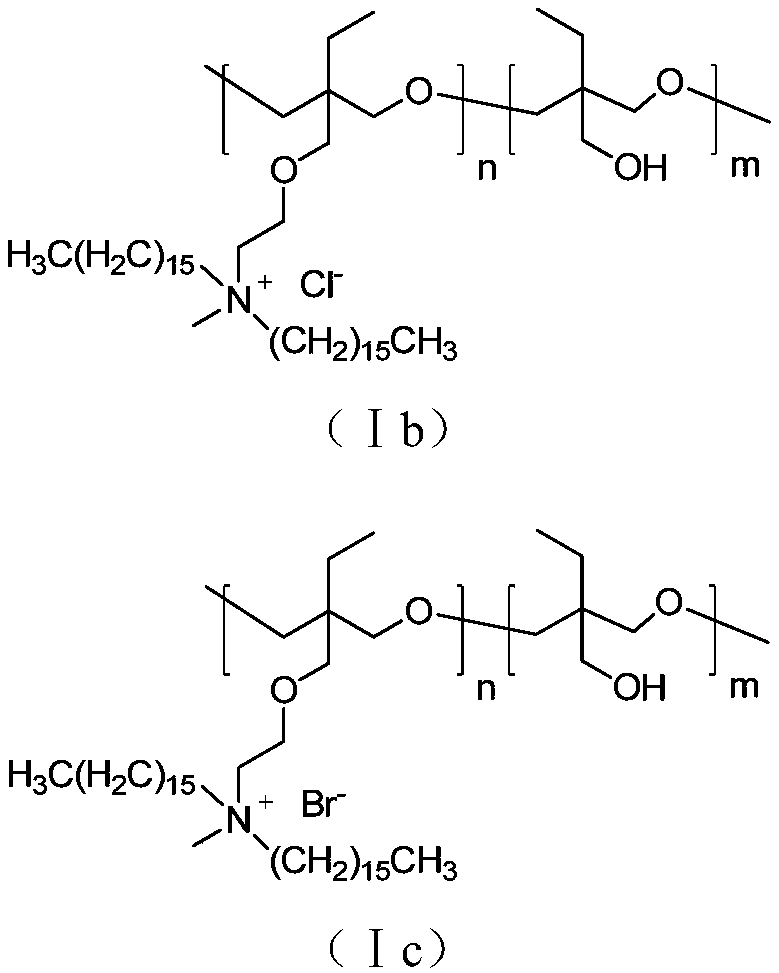
Side chain type water-soluble polyquaternium and preparation method thereof
A technology of water-soluble poly and quaternary ammonium salts, applied in the fields of botanical equipment and methods, chemical instruments and methods, and preparation of organic compounds, can solve the problems of poor reaction selectivity and high cost, and achieve less waste and strong operability. , The preparation method is simple and controllable
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1: Preparation of 3-ethyl-oxetane-3-methoxyethyl didecyl tertiary amine
[0026] In a 500mL four-neck flask equipped with mechanical stirring, add 7.6g ethanolamine, 44.0g chlorodecane, 220mL tetrahydrofuran, 30.4g triethylamine, 0.2g potassium iodide, heat to 50-60°C for 8 hours, TLC detection shows After the reaction is completed, the temperature of the reaction solution is lowered to 25-30° C., and then 33.7 g of 3-p-toluenesulfonyloxymethyl-3-ethyl-oxetane and 12.6 g of triethylamine are added, and the reaction is kept for 24 hours. The reaction solution was added with water and stirred, then extracted with ethyl acetate, the combined ethyl acetate layers were washed and precipitated, the residue was recrystallized by adding 50% ethanol, and after drying, 39.5g of light yellow needle-shaped crystals were obtained, the yield was 72%, and the content was ≥98.0%. . 1 H NMR (CDCl 3 )δ:4.23~4.51(m,4H),3.82(s,2H),3.35(t,2H),2.63(t,4H),2.28(t,2H),1.64(m,2H),1....
Embodiment 2
[0027] Embodiment 2: Preparation of 3-ethyl-oxetane-3-methoxyethyl dihexadecyl tertiary amine
[0028] In a 500mL four-neck flask equipped with mechanical stirring, add 7.6g ethanolamine, 65.0g hexadecane chloride, 180mL N,N-dimethylformamide, 30.4g triethylamine, 0.3g potassium iodide, and heat to 50~ React at 60°C for 12 hours, TLC detection shows that the reaction is complete, lower the temperature of the reaction solution to 25-30°C, then add 33.7g 3-p-toluenesulfonyloxymethyl-3-ethyl-oxetane and 12.6g Triethylamine, heat preservation reaction for 24 hours, the reaction solution was added with water and stirred, then extracted with ethyl acetate, the combined ethyl acetate layers were washed and precipitated, the residue was recrystallized by adding 95% ethanol, and after drying, 47.5g light yellow powder was obtained. The rate is 63%, and the content is ≥98.0%. 1 H NMR (CDCl 3 )δ: 4.20~4.48(m,4H),3.79(s,2H),3.23(t,2H),2.45(t,4H),2.16(t,2H),1.52(m,2H),1.28~1.51 (m,56H),...
Embodiment 3
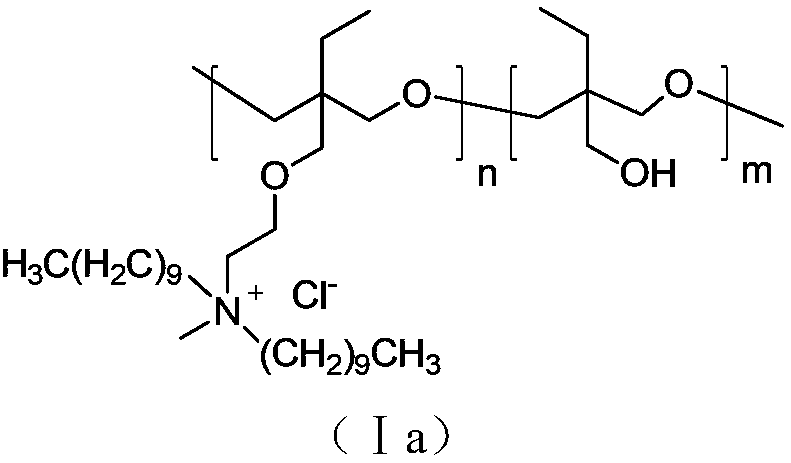
[0029] Embodiment 3: the preparation of compound (Ia)
[0030] In a 250mL four-necked bottle equipped with mechanical stirring and nitrogen protection device, add 0.70g of boron trifluoride ether, 0.16g of ethylene glycol and 25mL of anhydrous dichloromethane, cool down to 0-5°C, and keep dropping at this temperature Add 27.8 g of 3-ethyl-oxetane-3-methoxyethyldidecyl tertiary amine and 22.0 g of 3-ethyl-3-hydroxymethyl-oxetane to 100 mL of dichloromethane Solution, continue to stir at this temperature for 15 hours after the dropwise addition is completed, add 1g deionized water, 2g anhydrous sodium sulfate and 10g diatomaceous earth to the reaction solution, filter after stirring for half an hour, add dichloromethane to the filtrate Transfer 220mL of deionized water into a 500mL four-necked bottle equipped with a mechanical stirring and ventilating device, feed in methyl chloride gas under stirring, continue stirring for 1 hour after the ventilating is completed, then feed in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com