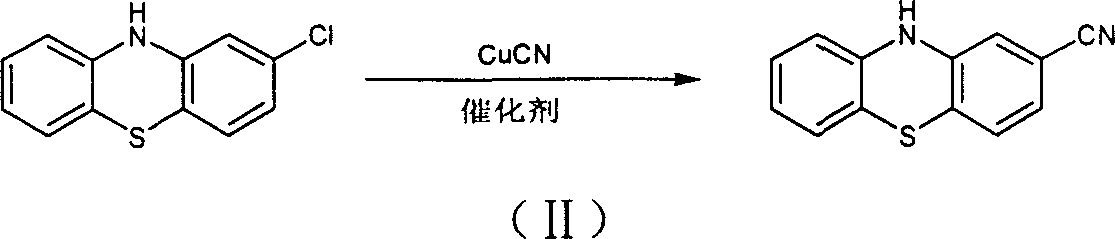
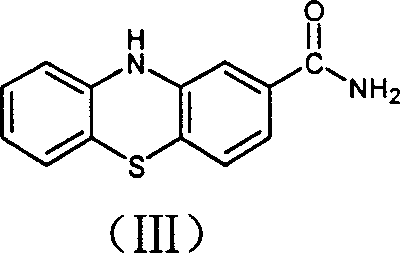
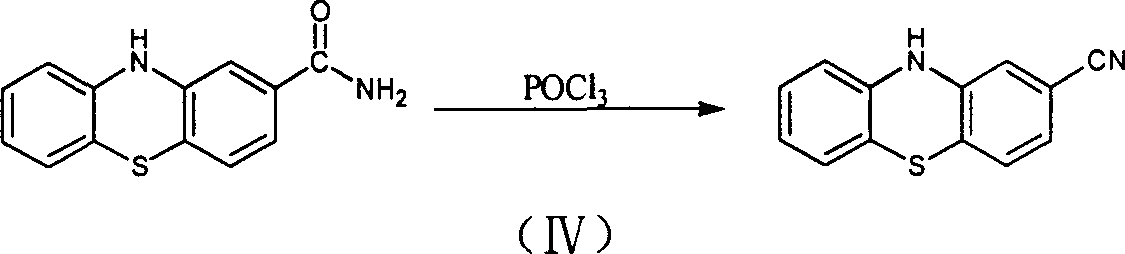Preparation of 2-cyanophenthiazine
A technology of phenothiazine and chlorophenothiazine, which is applied in the field of preparation of organic heterocyclic compounds, can solve the problems of complicated operation, decreased yield, increased industrial cost, etc., and achieves the effect of simple operation and reduced content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0024] Add 40 grams of 2-chlorophenothiazine, 20 grams of cuprous cyanide, 33 grams of potassium iodide and 50 milliliters of N-methylpyrrolidone into the reaction vessel, stir and raise the temperature to reflux, the reflux temperature is about 245-265 ° C; reflux reaction for 3 hours After cooling down to below 60°C, slowly add 500 ml of water and cool down to below 25°C, filter, rinse the solid with 150 ml of water; dry the solid under vacuum at 100°C for 2 hours; add the dried solid and 5 g of activated carbon into 600 ml of ethyl acetate, reflux for 1 hour, heat filter to remove insoluble impurities, and evaporate the solvent to obtain 35 g of 2-chlorophenothiazine crude product with a purity of 92.5%, of which amide impurities account for 5.6%. .
[0025] Add 35 grams of 2-chlorophenothiazine crude product to 70 milliliters of N, N-dimethylformamide, add 1.75 grams of phosphorus oxychloride, stir vigorously for 15 minutes; slowly add 500 milliliters of 2% sodium carbonat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com