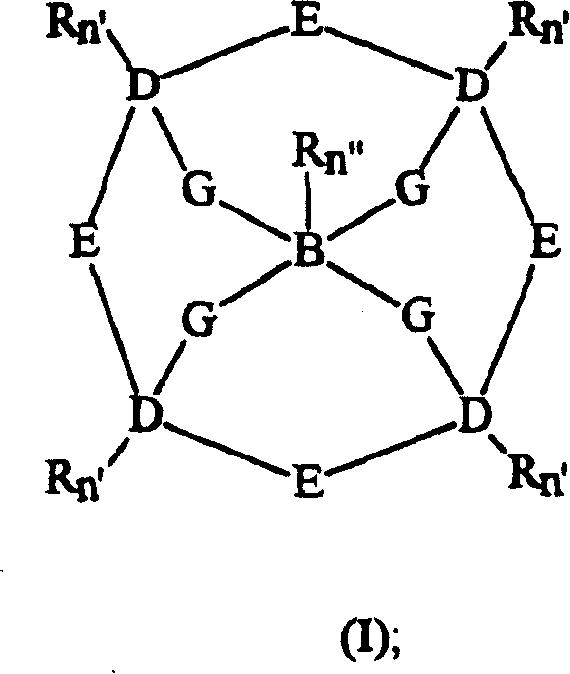
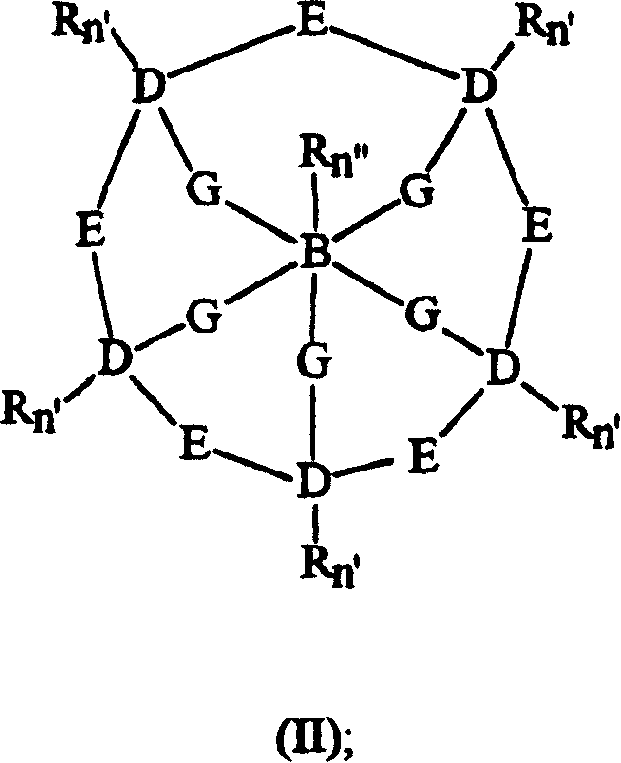
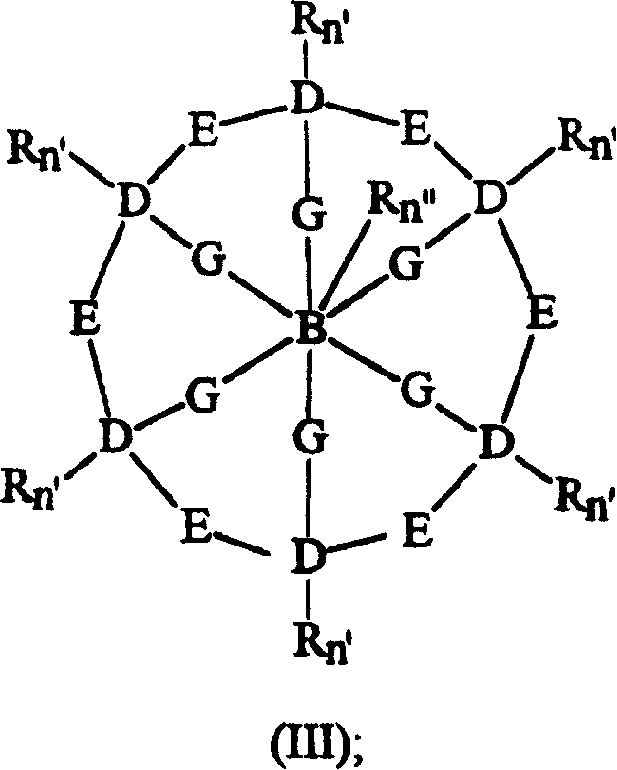
Bleach compositions containing metal bleach catalyst, and bleach activators and/or organic percarboxylic acids
A technology of bleach activator and bleach catalyst, applied in the direction of organic/inorganic compound composition, organic washing composition, detergent composition, etc., can solve the problems of finding and rarely reporting cross-linking bridges
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0345] Example 1 - [Mn(Bcyclam)Cl 2 ]Synthesis
[0346]
[0347] (a) Method 1
[0348] "Bcyclam" (5,12-dimethyl-1,5,8,12-tetraazabicyclo[6.6.2]hexadecane) was developed by G.R.Weisman et al., in Journal of the American Chemical Society (J. Amer. Chem. Soc.), (1990), 112 , Prepared by the synthetic method described in 8604. Dissolve Bcyclam (1.00 g, 3.93 mmol) in anhydrous CH 3 CN (35ml, from CaH 2 distilled). The solution was then evacuated at 15 mm pressure until CH 3 CN started to boil. The flask was then connected to atmospheric pressure Ar. This degassing step was repeated 4 times. Under Ar atmosphere, adding according to H.T.Witteveen et al. Journal of Inorganic Nuclear Chemistry (J. Inorg. Nucl. Chem.) (1974), 36 , Mn(pyridine) synthesized by the method in 1535 2 Cl 2 (1.12 g, 3.93 mmol). The cloudy reaction solution slowly started to turn black. After stirring overnight at room temperature, the reaction solution turned dark brown with su...
Embodiment 2
[0351] Embodiment 2-[Mn(C 4 -Bcyclam)Cl 2 ] synthesis, where C 4 -Bcyclam=5-n-butyl
[0352] -12-Methyl-1,5,8,12-tetraazabicyclo[6.6.2]hexadecane
[0353]
[0354] (a)C 4 -Synthesis of Bcyclam
[0355]
[0356] Will press H.Yamamoto and K.Maruoka's Journal of the American Chemical Society (J. Amer. Chem. Soc.), (1981), 103 , Tetracycloadduct I (3.00 g, 13.5 mmol) prepared by the method described in 4194 was dissolved in anhydrous CH 3 CN (50ml, from CaH 2 distilled). Under Ar atmosphere, 1-iodobutane (24.84 g, 135 mmol) was added to the stirred solution. The solution was stirred at room temperature for 5 days. 1-Iodobutane (12.42 g, 67.5 mmol) was added and the solution was stirred at room temperature for an additional 5 days. Under these conditions, by 13 C-NMR shows, I Completely 1-iodobutane-alkylated. Methyl iodide (26.5 g, 187 mmol) was added and the solution was stirred at room temperature for an additional 5 days. The reaction was filtered u...
Embodiment 3
[0360] Example 3. [Mn(Bz-Bcyclam)Cl 2 ], wherein Bz-Bcyclam=5-benzyl-
[0361] 12-Methyl-1,5,8,12-tetraazabicyclo[6.6.2]hexadecane
[0362]
[0363] (a) Synthesis of Bz-Bcyclam
[0364] Using C similar to that described in Example 2(a) above 4 -Bcyclam synthetic method to synthesize the ligand, just replace 1-iodobutane with benzyl bromide. 13 C NMR (CDCl 3 ) 27.6, 28.4, 43.0, 52.1, 52.2, 54.4, 55.6, 56.4, 56.5, 56.9, 57.3, 57.8, 60.2, 60.3, 126.7, 128.0, 129.1, 141.0ppm. Mass spectrometry (MH + , 331).
[0365] (b)[Mn(Bz-Bcyclam)Cl 2 ]Synthesis
[0366] [Mn(C 4 -Bcyclam)Cl 2 ] to prepare the complex, just replace C with Bz-Bcyclam 4 -Bcyclam. Ion spray mass spectrometry analysis showed a corresponding [Mn(Bz-Bcyclam)(formate)] at 430mu + a main peak of .
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| pour point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com