Compsns. comprising comjugates of stable, active, human ob protein with antibody FC chain and methods
A protein, immunoglobulin technology, applied in drug combinations, animal/human proteins, antibody mimics/scaffolds, etc., can solve the problems of impairing patient compliance, inconvenience, and expensive storage.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Embodiment 1: the preparation of amorphous OB protein suspension
[0063] This example illustrates the preparation of a suspension of human OB protein of the present invention. Suspensions of amorphous human OB protein were prepared by precipitation with zinc salts. A liquid with a final pH of 6.0 to pH 8.0 and a concentration of 100 mg protein / ml was obtained. A control composition of a pH 4.0 human OB protein solution is also given.
[0064] combination
[0065] Protein component: Recombinant methionyl human OB protein ("rmetHu-leptin") described in SEQ ID NO: 4 of PCT publication WO 96 / 05309, amino acid number starting at position 22 (Val) and amino acid number 167 end with a methionyl residue at the N-terminus.
[0066] Precipitating agent: zinc chloride
[0067] Buffers: Tris, MES, and Pipes
[0068] Final pH: 6.0-8.0
[0069] Preparation method: Recombinant methionyl human OB protein (“rmetHu-leptin”) solution was concentrated by injection in water to about...
Embodiment 2
[0071] Example 2: Preparation of crystallized OB protein suspension
[0072] This example illustrates the preparation of a crystalline OB protein suspension of the invention.
[0073] Protein component: Recombinant methionyl human OB protein was used as in Example 1 above.
[0074] Method: rmetHu-leptin at a concentration of 15 mg / ml in 1 mM HCl was incubated with 4M NaCl, 100 mM Tris, pH 8.5, 2% v / v ethanol in a 1:1 ratio at 4°C. Crystals formed spontaneously by slowly adjusting the temperature between 14°C and 25°C for several hours, maintaining this temperature for at least 2 hours depending on the duration of incubation to the final temperature. The crystals are harvested by centrifugation, suspended in a suitable crystallization stabilizing solvent, and the "mother liquor" (ie, the liquid in which the crystals grow) is replaced by a more stable solvent by injection. A suitable substituting solvent is 20-25% polyethylene glycol (having a molecular weight of about 4000 Da...
Embodiment 3
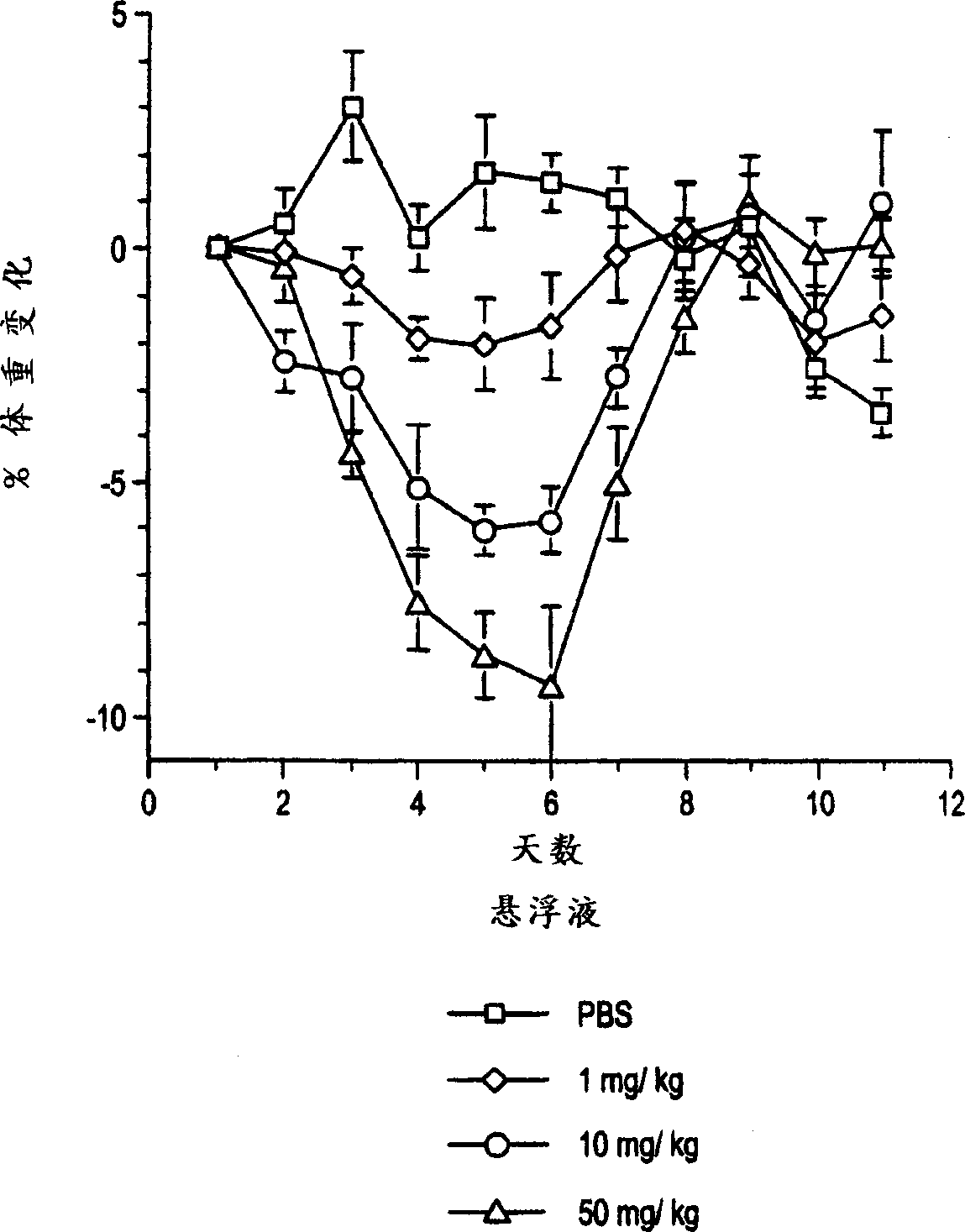
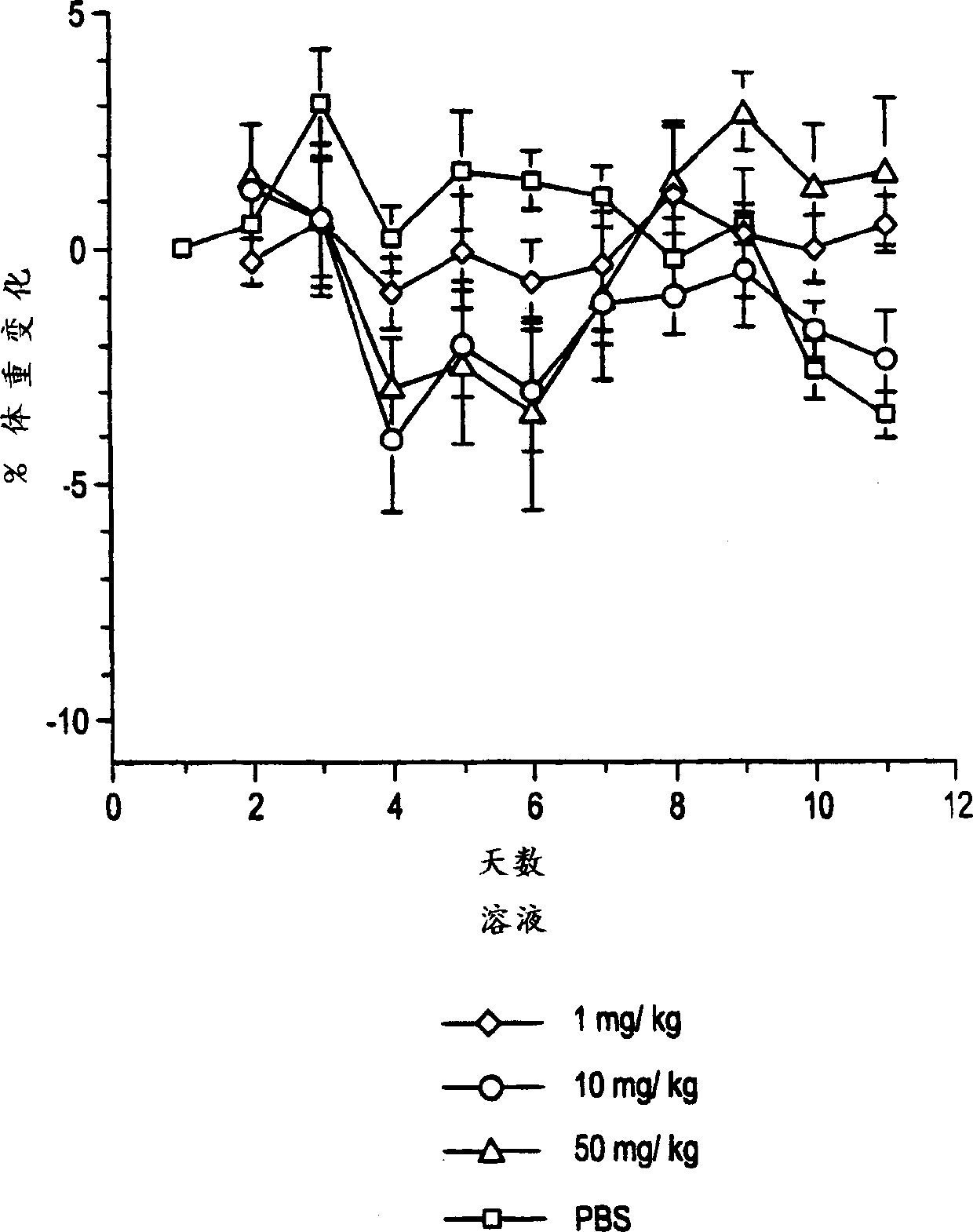
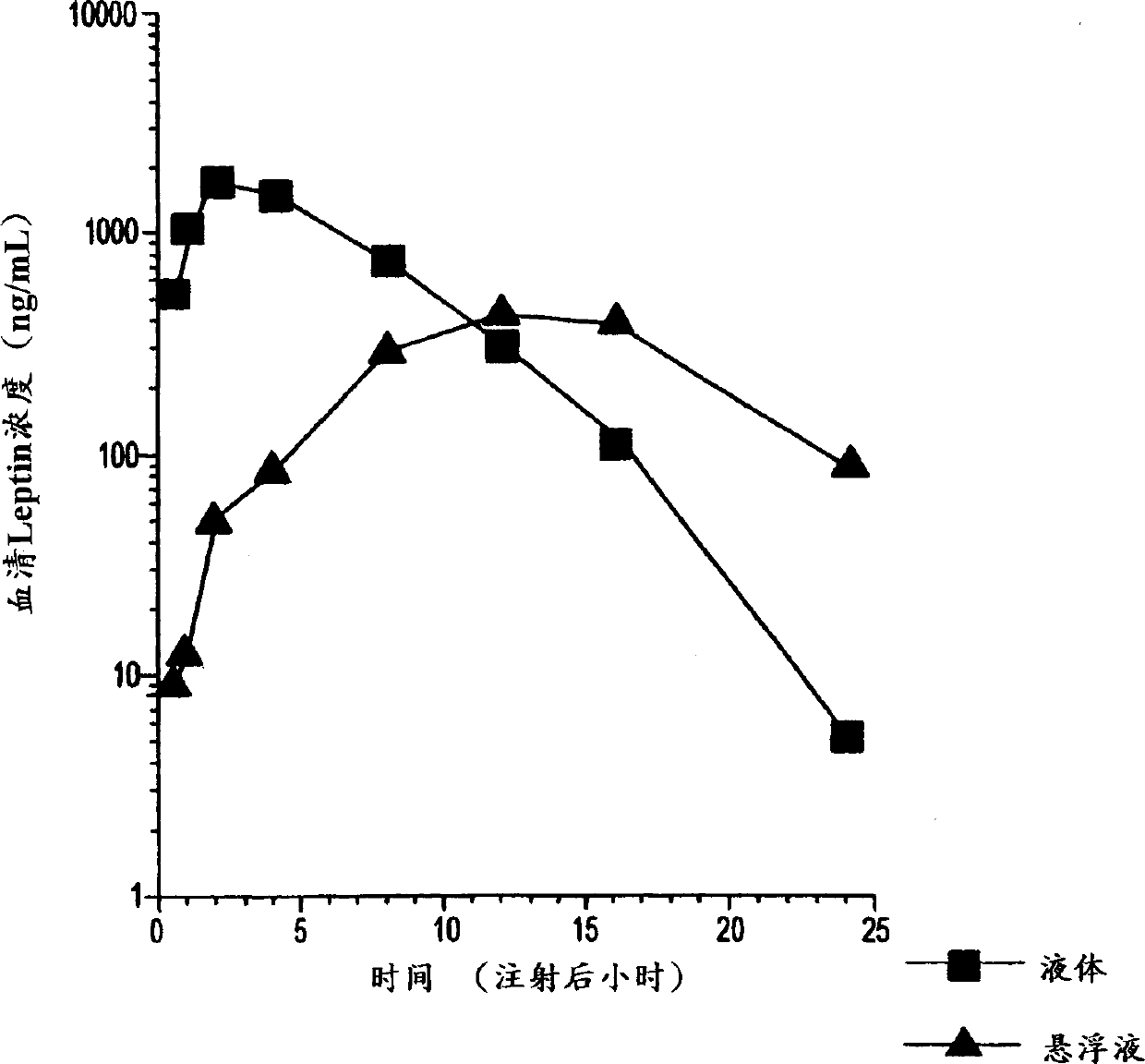
[0075] Example 3: Improved dose response of OB protein suspension compared to OB protein solution
[0076] This example illustrates that the OB protein suspension of the present invention is more effective than the OB protein in solution. Normal lean mice were injected with 1, 10 and 50 mg protein suspension of the present invention or OB protein solution of the same dose for 5 consecutive days. Mice given the suspension lost more weight than mice given an equivalent dose of the solution formulation, calculated per unit weight of the amount of OB protein given. The results are illustrated in Figures 1A and 1B. Figure 1A shows the percentage change in body weight when the suspension of Example 1 was administered. Figure 1B shows the percentage change in body weight when the control solution of Example 1 was administered.
[0077] The results demonstrate that the suspension of the present invention is more effective than the protein given in solution at the same dose. While ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com