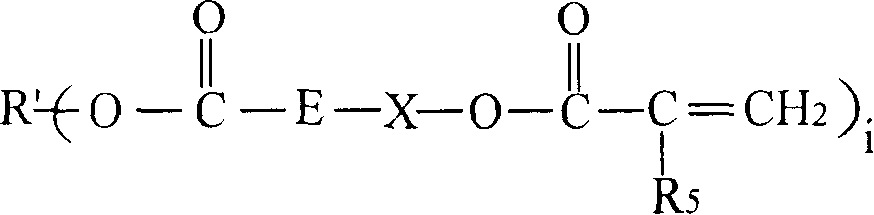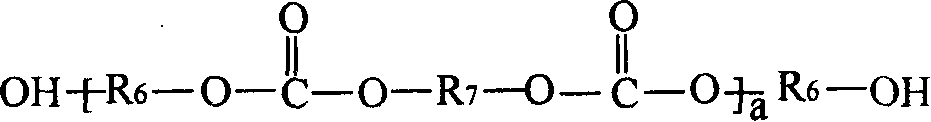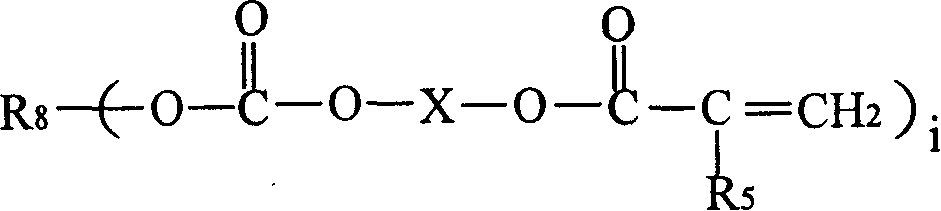Photochromic polymerizable compositions
A polymer composition and photochromic technology, applied in the direction of color-changing fluorescent materials, optics, optical components, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0120] The preparation of the chloroformate intermediate and the subsequent reaction with the hydroxyl (meth)acrylate can be carried out by methods known in the prior art. As those skilled in the art know, the reaction of chloroformate groups with hydroxyl functional (meth)acrylates is usually carried out in the presence of acid scavengers, such as alkali metal hydroxides, followed by washing and separating the final A mixture of polyol ((meth)acryloyl carbonate) monomers. In step (b), the molar equivalent ratio of the hydroxyl functional (meth)acrylate to the chloroformate group in the chloroformate intermediate mixture may be less than 1:1. In a non-limiting embodiment, it is at least It is 1:1 (that is: all the chloroformate groups react with the hydroxyl (meth)acrylate). In step (b) of the method, the molar equivalent ratio of the hydroxyl (meth)acrylate to the chloroformate group can be from 1:1 to 1.5:1.0, for example 1.1:1.0.
[0121] In another non-limiting embodiment, the...
Embodiment 1-14
[0214] Table 6 lists the monomers in Examples 1-14 in weight percent. The example is prepared by the following steps: add the monomer composition listed in Table 6 to a suitable container equipped with a stirring device, and stir for 1 hour after the following additives are added: Add 0.15wt% can be obtained by 3M FC-431 fluorocarbon surfactant and 28.0wt% of the photochromic component in Table 5, both weight percentages are based on the total weight of the monomer.
[0215] Example#
1
2
3
4
5
6
7
8
9
10
11
12
13
14
BPA2EO DMA(n)
45
40
30
45
45
45
70
70
70
70
70
70
70
70
Component 1
35
40
30
8.75
17.5
26.25
30
15
0
15
10
0
0
0
Component 2
0
0
0
26.25
17.5
8.75
0 ...
Embodiment 15
[0222] The accelerated aging photochromic optical fatigue percentage test (AWPPPF test) involves preparing the lens in the AC part before or after the aging of the G part, coating the lens with the polymerizable composition in the D part, and measuring the coated lens in the E part Fischer micro-hardness, determine the photochromic performance and fatigue in part F.
[0223] The polymerizable composition of the present invention was added to the coating compositions in Examples 1-8, 10, and 11. Other polymerizable compositions that do not contain the polymerizable composition of the present invention, such as the compositions of Examples 9, 12, 13, and 14, were also tested. Compare the photochromic properties of the coatings with comparable Fischer micro-hardness levels and the results of fatigue measurements to determine whether these parameters have increased or decreased. Since it is generally known that the performance of photochromic compounds can be accelerated in a softer p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| microhardness | aaaaa | aaaaa |
| microhardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com