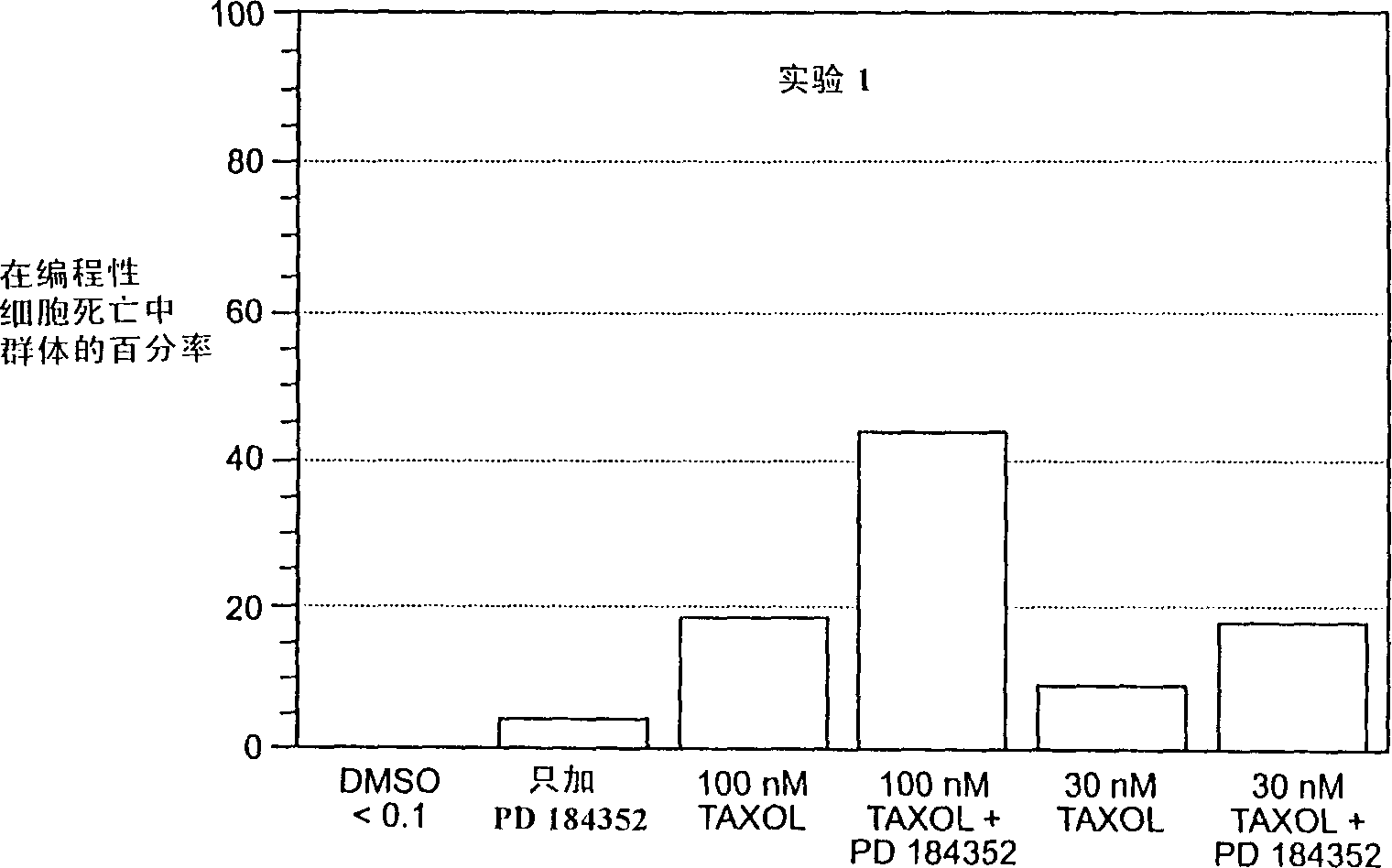
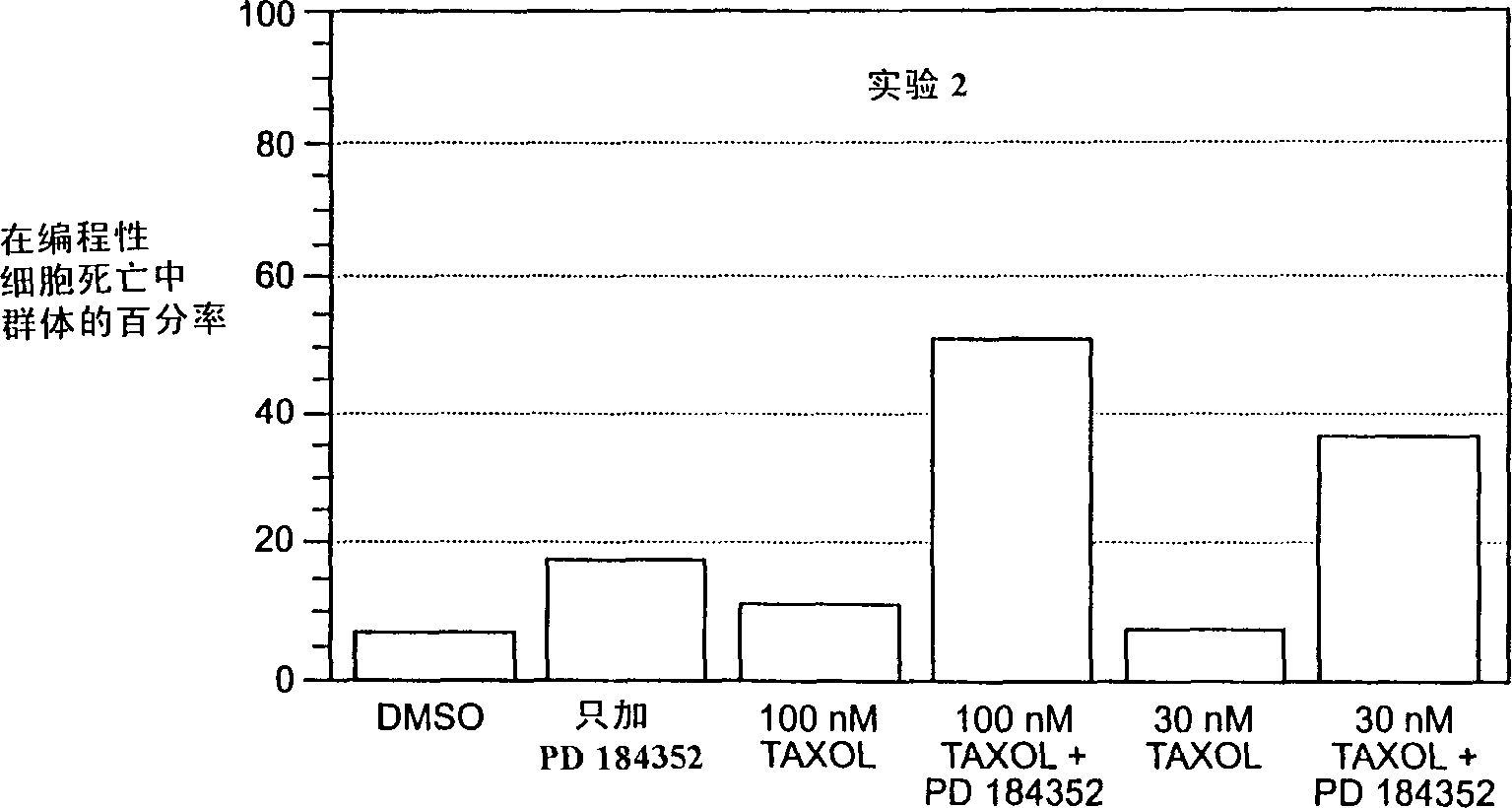
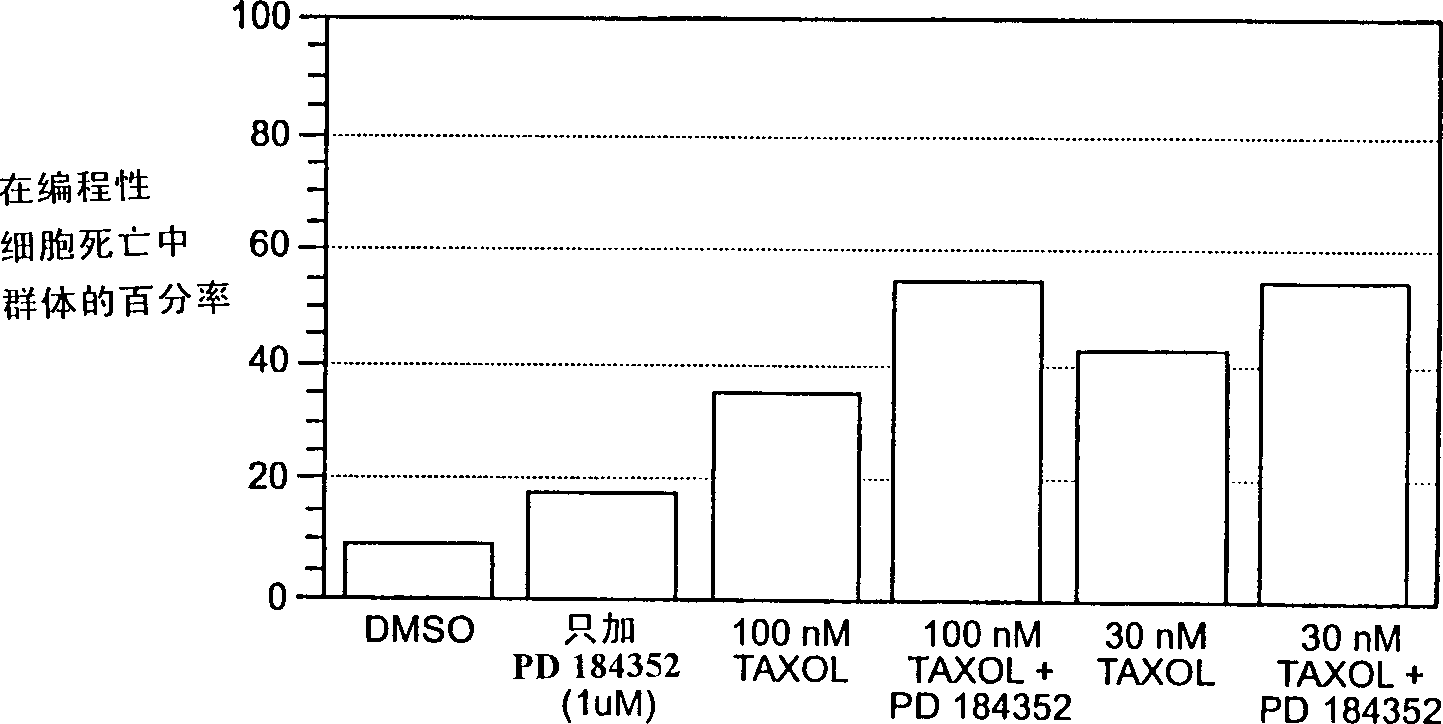
Combination chemotherapy
A composition and inhibitor technology, applied in the directions of drug combinations, active ingredients of heterocyclic compounds, active ingredients of amides, etc., can solve the problems of interphase and mitotic function inhibition, microtubules not functioning normally, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 14
[0071] Example 14-fluoro-2-(4-iodo-2-methylphenylamino)benzoic acid
[0072] Add 10 ml (0.020 mol) of 2.0 M lithium diisopropylamide in THF / Solution in heptane / vinylbenzene (Aldrich). The resulting green suspension was stirred vigorously for 15 minutes, then a solution of 1.00 g (0.00632 mol) of 2,4-difluorobenzoic acid in 10 mL of tetrahydrofuran was added. The reaction temperature was allowed to rise slowly to room temperature, where it was stirred for 2 days. The reaction mixture was concentrated. A 10% aqueous hydrochloric acid solution was added to the concentrate, and the solution was extracted with dichloromethane. The organic phase was dried (MgSO 4 ), then boil on a jet bath to reduce volume and cool to room temperature. Off-white fibers were collected by vacuum filtration, rinsed with hexane and dried in a vacuum oven (76 °C; about 10 mmHg) to give 1.10 g (47%) of the desired material; mp 224-229.5 °C; 1 H NMR (400MHz; DMSO): δ9.72(s, 1H), 7.97(dd, 1H, J=7.0, ...
Embodiment 2-30
[0074] According to the general procedure of Example 1, the following benzoic acid and salts of formula (I) were prepared. Melting point of Example No. compound ℃ 2 3,4,5-trifluoro-2-(4-iodo-2-methyl-phenylamino)-benzoic acid 206-210 3 3,4-difluoro-2-(4 -Iodo-2-methyl-phenylamino)-benzoic acid 240.5-244.5 4 5-bromo-3,4-difluoro-2-(4-iodo-2-methyl-phenylamino)-benzoic acid 259.5 -262 5 5-chloro-2-(2-(chloro-4-iodo-phenylamino)-benzoic acid 255-260 6 5-chloro-2-(4-iodo-2-methyl-phenylamino) - Benzoic acid 234-238 7 5-chloro-2-(4-iodo-2-methyl-phenylamino)-sodium benzoate 310-320 decomposition 8 5-bromo-2-(4-iodo-2-methyl -phenylamino)-5-nitro-benzoic acid 239.5-240 9 2-(2-chloro-4-iodo-phenylamino)-5-nitro-benzoic acid 289-29310 4-fluoro-2-( 3-fluoro-4-iodo-2-methyl-phenylamino)-benzoic acid 233-23511 2-(4-iodo-2-methyl-phenylamino)-5-nitro-benzoic acid 264-26712 2-(2-fluoro-4-iodo-phenylamino)-5-nitro-benzoic acid 256-25813 2-(4-bromo-2-methyl-phenylamino)-4-fluoro-benzoic a...
Embodiment 315
[0075] Example 315-Chloro-N-(2-hydroxyethyl)-2-(4-iodo-2-methyl-phenylamino)-benzamide
[0076] To a stirred solution containing 0.1020 g (0.2632 mmol) of 5-chloro-2-(4-iodo-2-methyl-phenylamino)-benzoic acid, 0.1 ml (1.7 mmol) ethanolamine and 0.05 ml (0.29 0.15 g (0.29 mmol) of solid PyBOP powder was added directly to a solution of 1:1 (v / y) tetrahydrofuran-dichloromethane solution in 5 mL of diisopropylethylamine. The reaction mixture was stirred overnight at room temperature. Solvent was removed in vacuo. The crude residue was partitioned between diethyl ether (50 mL) and 10% aqueous hydrochloric acid (50 mL). The organic phase was washed with 10% aqueous NaOH (50 mL), dried (MgSO 4 ) and concentrated under vacuum to give a yellow-brown oil, which was crystallized from hexane-ether to give 0.0831 g (73%) of a green-yellow powder; mp 120-121°C; 1 H NMR (400MHz; CDCl 3 ): δ9.11(s, 1H), 7.56 (d, 1H, J=1.4Hz), 7.46-7.41(m, 2H), 7.20(dd, 1H, J=8.9, 2.4Hz), 7.00(t, 2H, J=9...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com