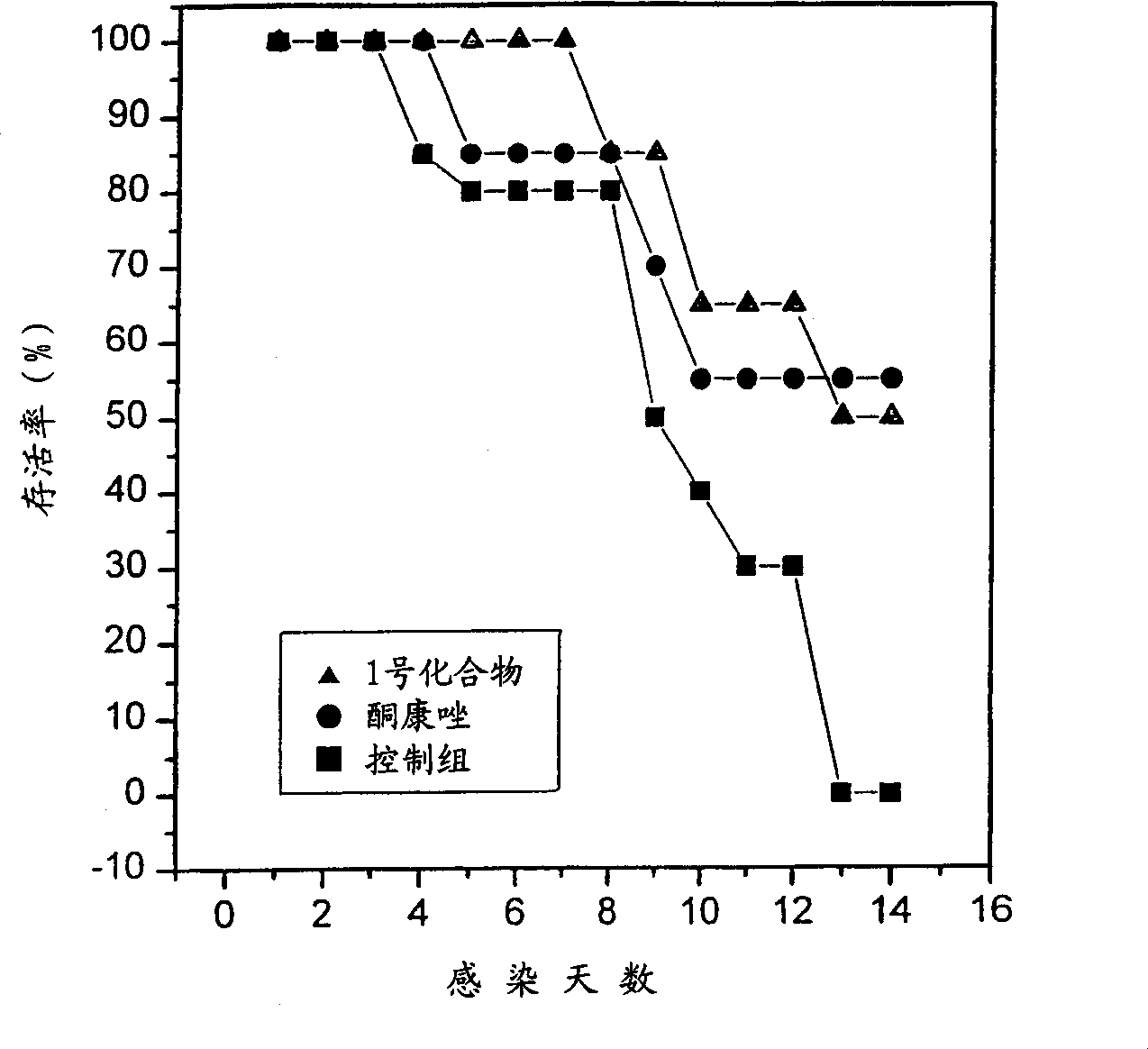
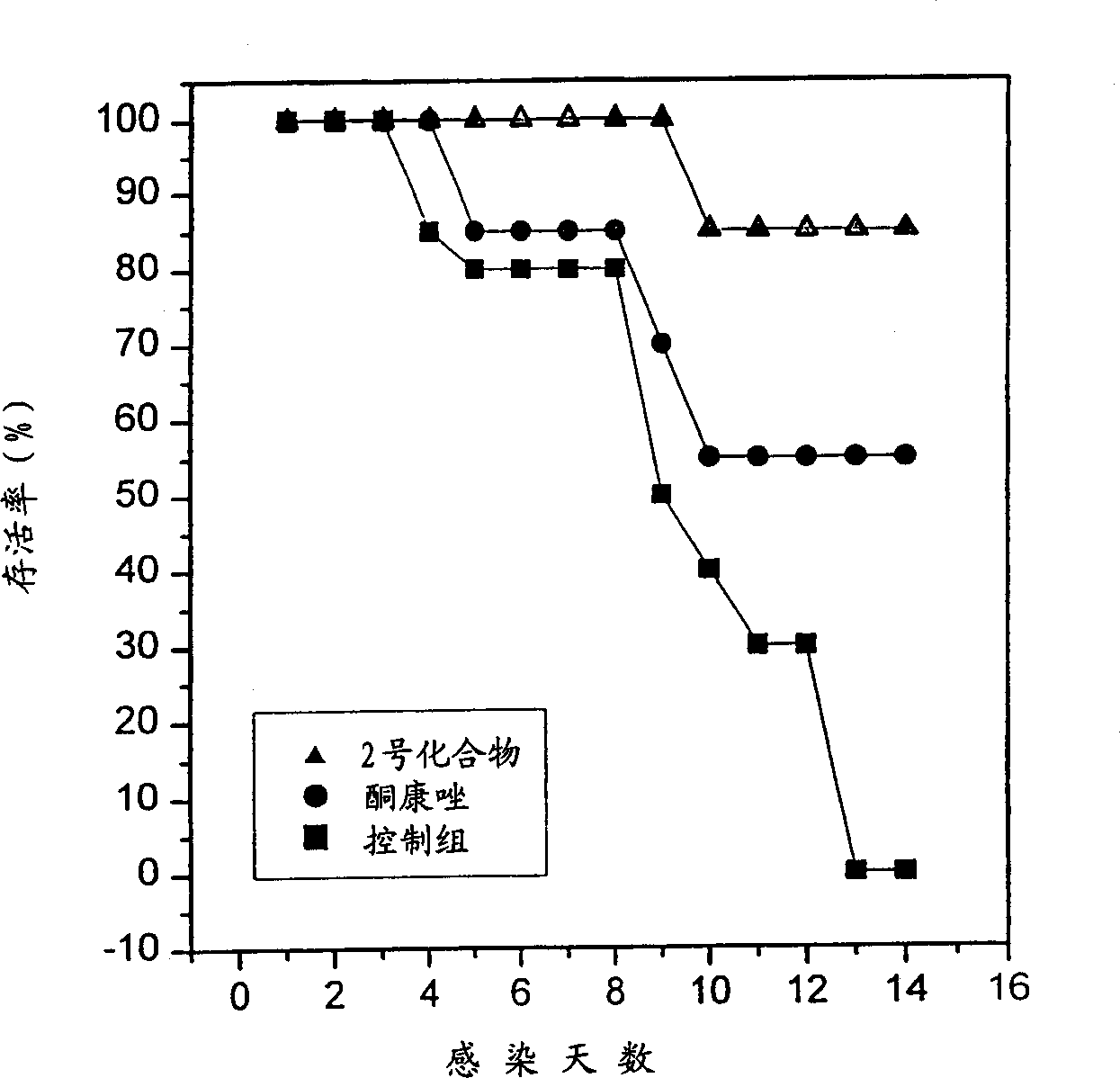
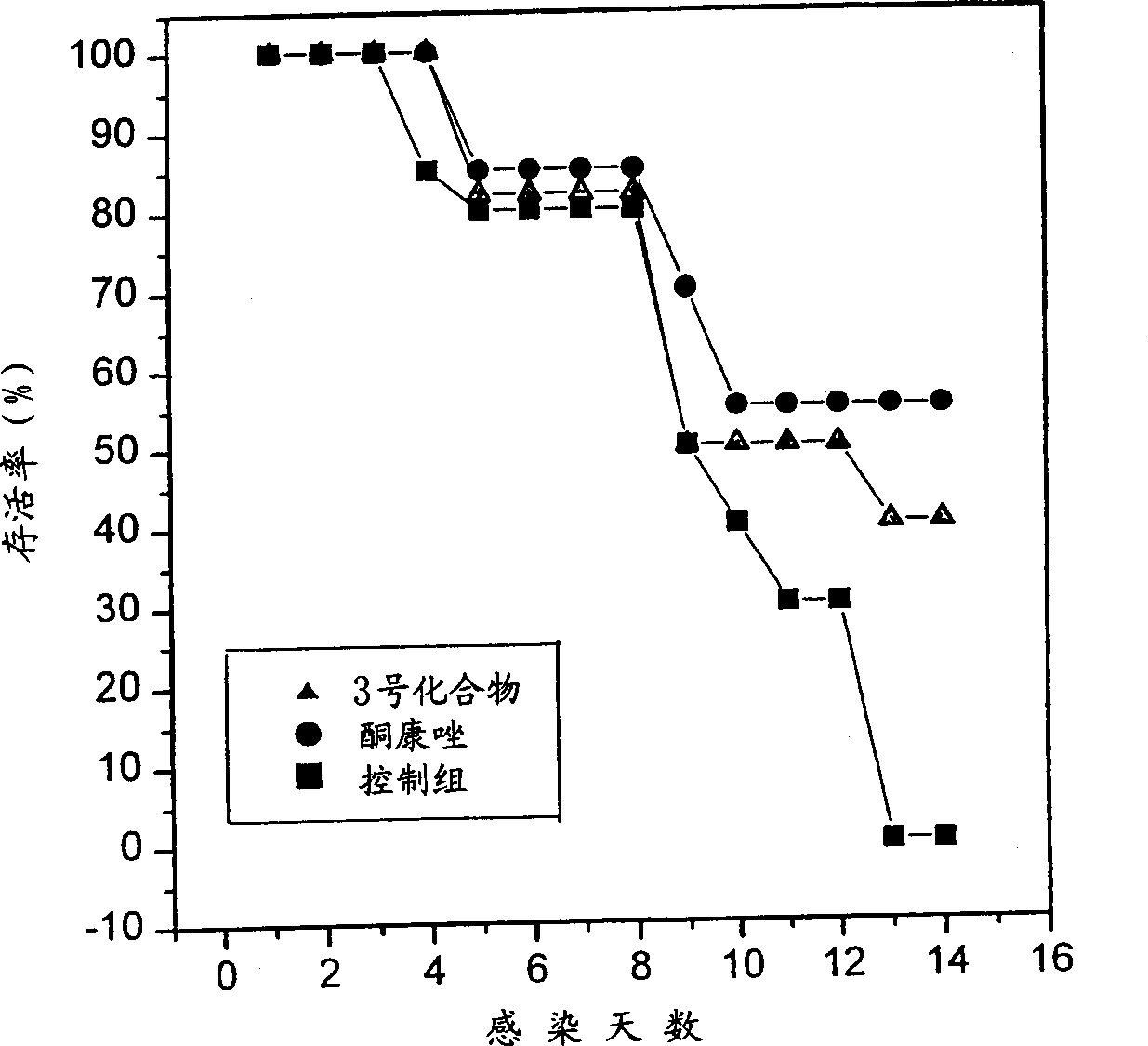
6.7-disubstituted-5,8-quinolinedione derivatives as antifungal agent
A kind of quinoline dione, antifungal technology, applied in 6 fields, can solve the problem of human pathology fungi antifungal activity experiment or mention and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0044] Example 1: 5-nitroso-8-hydroxyquinoline hydrochloride
[0045] Add NaNO to a mixture 2 Aqueous solution, of which water is 100ml, NaNO 2 It is 30g, the adding time is 1 hour, the temperature is 0-4℃, the mixture is composed of the following materials: 8-hydroxyquinoline (58g, 0.4 mol) in distilled water, concentrated hydrochloric acid (75ml) and ice ( 200g). During the whole night, the reactant was kept at 0°C, and then filtered by washing with cold water to obtain 5-nitroso-8-hydroxyquinoline hydrochloride (95%).
example 2
[0046] Example 2: 5-amino-8-hydroxyquinoline dihydrochloride
[0047] To a mixture consisting of water (160ml) and 5N-NaOH (260ml), 5-amino-8-hydroxyquinoline chloride (40g) was added and heated to 40°C. Add Na to the reaction mixture 2 S 2 O 4 (95g), the reaction temperature was increased to 75-80°C. The reaction mixture was cooled to 50°C, and 12N-HCL (250 ml) was added to the reaction mixture. The reaction mixture was then cooled to 0°C and filtered to obtain 5-amino-8-hydroxyquinoline dihydrochloride (34 g, 69%).
example 3
[0048] Example 3: 6,7-dihalo-5,8-quinolinedione of structural formula 2
[0049] After adding 5-amino-8-hydroxyquinoline dihydrochloride (9g) to hydrochloric acid (81g), the reaction mixture was heated to 60°C and NaClO was added 3 (4.5g). At a temperature of 50-60°C, the reaction mixture was stirred for 30 minutes, filtered, and recrystallized twice with butanol to obtain 6,7-dihalo-5,8-quinolinedione (90%) yellow Precipitate. Melting point: 221-222°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com