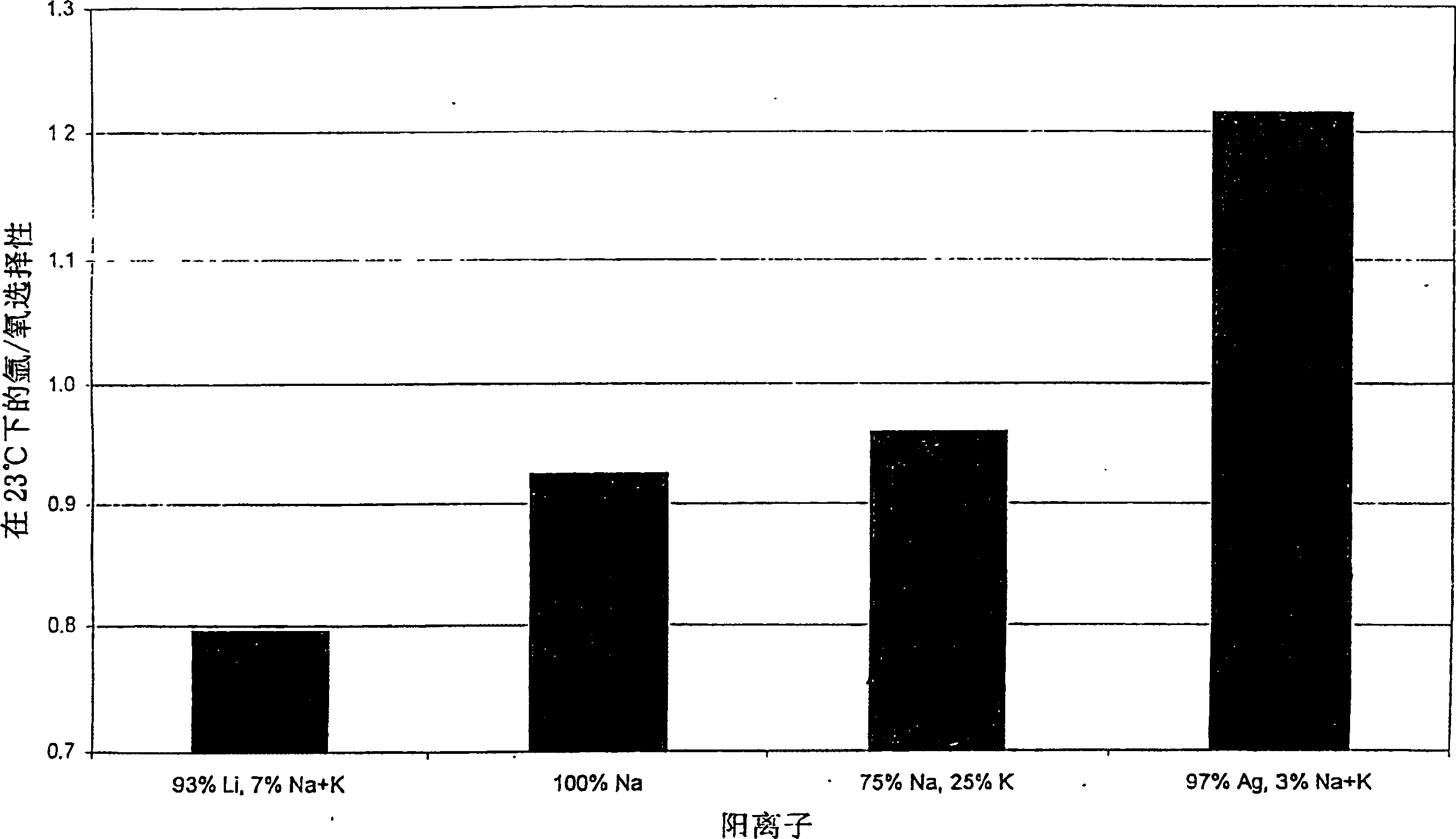
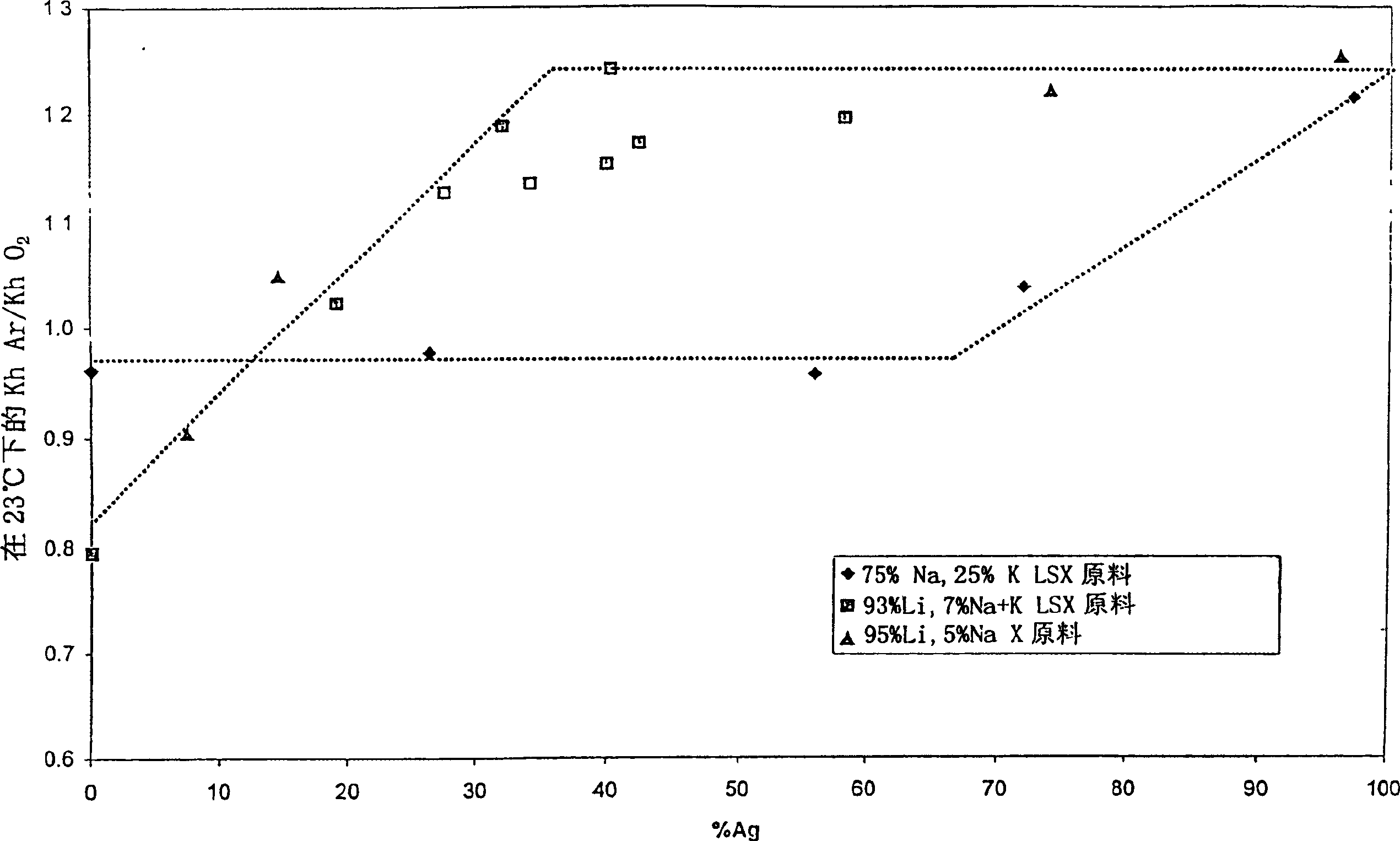
Argon/oxigen selective X-zeolite
A zeolite, selected technology, applied in the field of X-zeolite with argon/oxygen selectivity, can solve the problem of non-priority, and achieve the effect of low cost and enhanced argon/oxygen selectivity ratio
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0019] The crystalline zeolite materials described herein are suitable for adsorbing argon from a mixture comprising argon and oxygen. While many crystalline zeolitic materials are effective for separating argon from oxygen, they are not effective for separating argon from oxygen by adsorption techniques. The crystalline zeolite adsorbent described here and suitable for the separation of argon from oxygen consists of a silver-exchanged lithium-X zeolite with Li x Ag y m z The ion exchange subcomponent in the form of X, where 0.85≤x+y≤1, 0.2≤y≤0.7, 0.0≤z≤0.15, M represents one or more cations, and x, y, and z represent the total The number of copies that can be swapped. M may be one or more elements in cationic form selected from the group consisting of: alkali or alkaline earth metals, rare earths, transition metals, or Group IIIA metals. Preferably, M is one or more elements in the form of cations selected from Na, K, Cs, Mg, La, Ce, Ca, Al, or Zn. Preferably, the sorben...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com