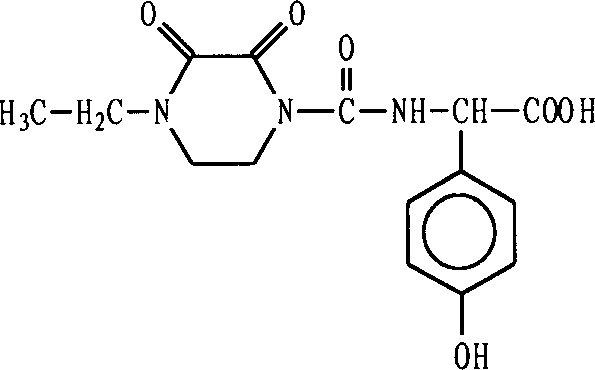
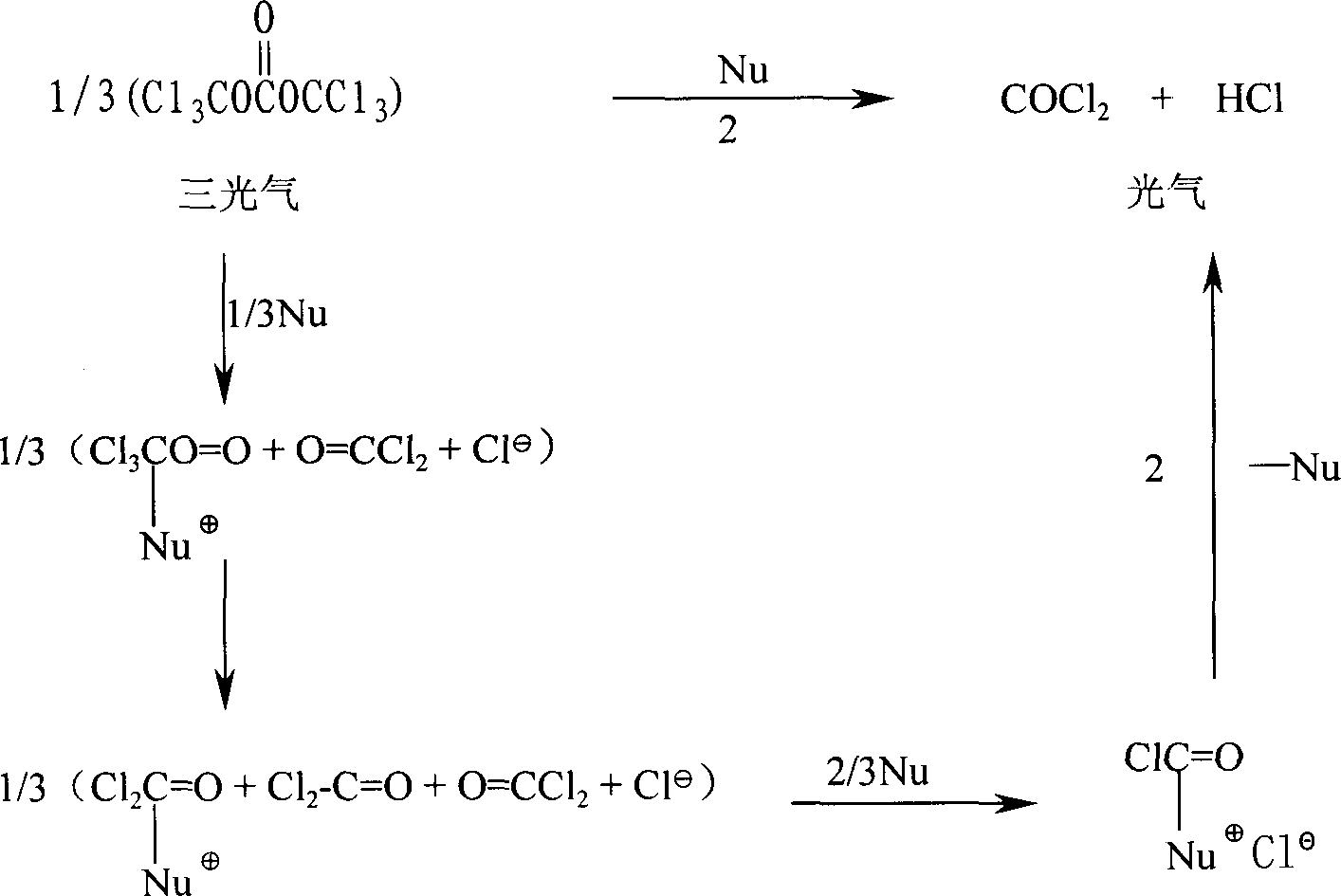HO-EPCP synthesizing method
A technology of HO-EPCP and synthesis method, which is applied in the fields of medicine and chemical industry, and can solve the problems of liquid phosgene transportation and storage hazards, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Dissolve 12.0 g of 4-ethyl-2,3-dioxopiperazine in 60 ml of CH 2 Cl 2 12.0 g of trimethylsilyl was added, and 10.0 g of triethylamine was added dropwise at -10°C. After the addition, the reaction was continued at this temperature for 2 hours to obtain siliconized oxygen piperazine (I).
[0023] Dissolve 10.0 g of triphosgene in 40 ml of CH 2 Cl 2 Add 1.0 g of triethylamine as an initiator, add (I) dropwise to the triphosgene solution within 2 hours at about -10°C, and gradually rise to room temperature after adding, this is oxypiperazine chloride (II) .
[0024] Add 12.0 g of p-hydroxyphenylglycine to 150 ml of CH 2 Cl 2 23.2 g of triethylamine was added under stirring, then 26.3 g of trimethylsilane was added dropwise at 10-15°C, and the reaction was continued for 2 hours after the addition was complete. This is siliconized p-hydroxyphenylglycine (III).
[0025] (II) was dripped into (III) at 15-20° C., and the reaction was continued for 3 hours. 200 ml of disti...
Embodiment 2
[0028] With 60.0 grams of piperazine under the same conditions of example 1, carry out silicon esterification with trimethylsilyl and triethylamine to obtain (I), add (I) to 50.0 grams of triphosgene in 3 hours at -15~-20 ℃ 200mlCH 2 Cl 2 In the solution, (initiated by adding 3.5 g of triethylamine in advance) after the addition, the stirring was continued for 2 hours, and gradually rose to room temperature to obtain (II).
[0029] In addition, 60.0 g of p-hydroxyphenylglycine was silicon-esterified under the same conditions as in Example 1 to obtain (III). The remaining operations were the same as in Example 1 to obtain 94.8 g of crude product HO-EPCP with a yield of 98.3%.
[0030] After refining, 86.2 g of white crystals were obtained, the HO-EPCP content was 99.97%, the yield was 89.4%, the melting point was 215.3-216.3°C, and the specific rotation α=-90.78°.
Embodiment 3
[0031] Embodiment 3 (contrast)
[0032] Dissolve 12.0 g of 4-ethyl-2,3-dioxypiperazine in 60 ml of CH 2 Cl 2 Add 12.0 grams of trimethylsilane, add 10.0 grams of triethylamine dropwise at -10°C, continue to stir at this temperature for 2 hours after the addition, gradually rise to 0°C, remove the Et produced by the reaction by filtration 3 N·HCl to obtain a siliconized oxygen piperazine solution (I).
[0033] Dissolve 10.0 g of triphosgene in 40 ml of CH 2 Cl 2 , without adding an initiator, directly drop (I) at about -10°C, and gradually rise to room temperature to obtain (II) after the addition. All the other operations are the same as in Example 1. 10.82 g (crude product) of HO-EPCP was obtained with a yield of 44.9%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| specific rotation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com