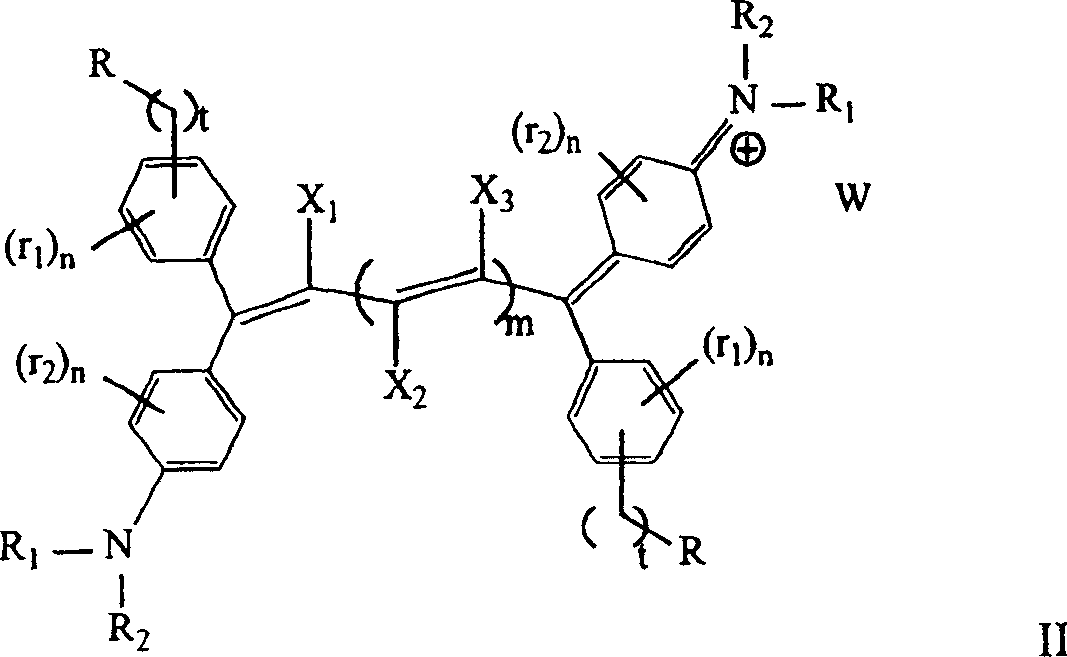
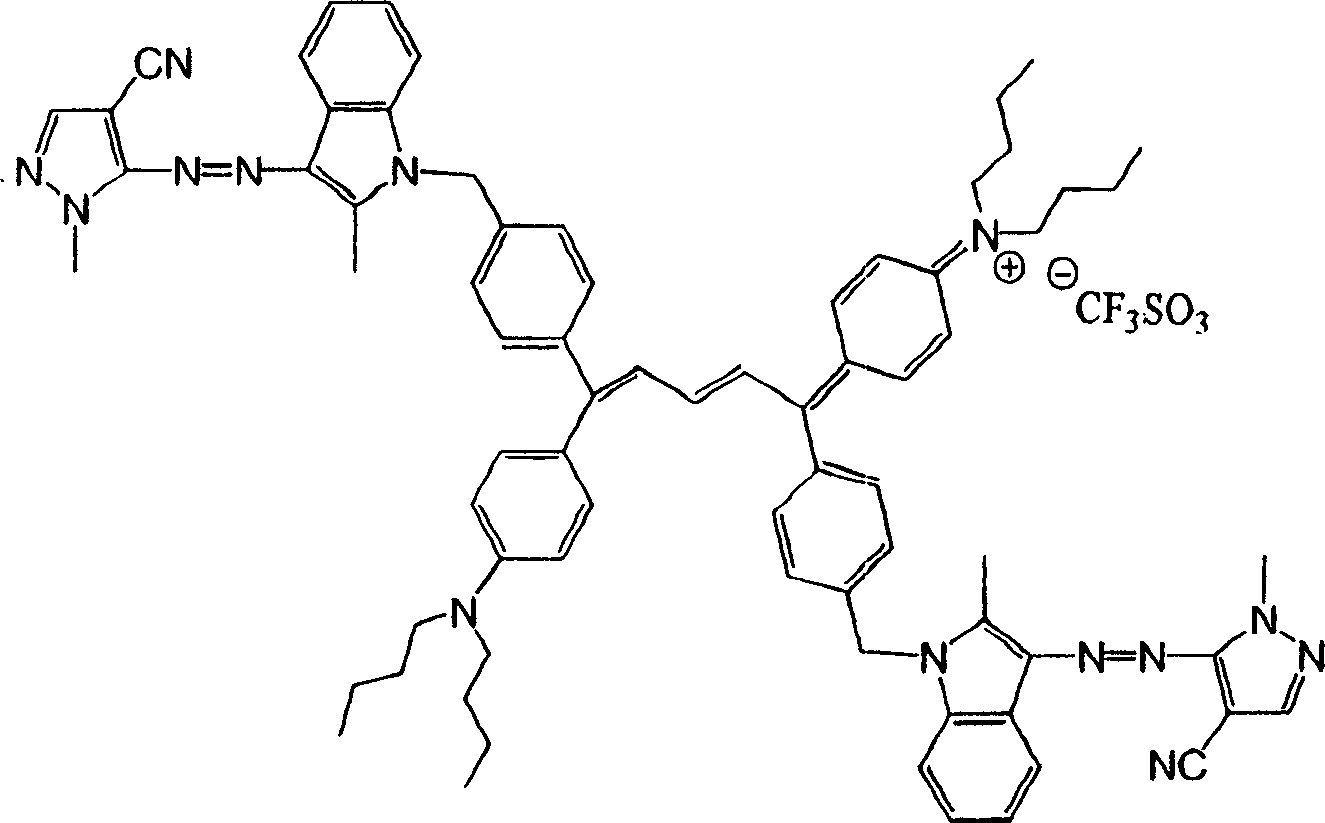
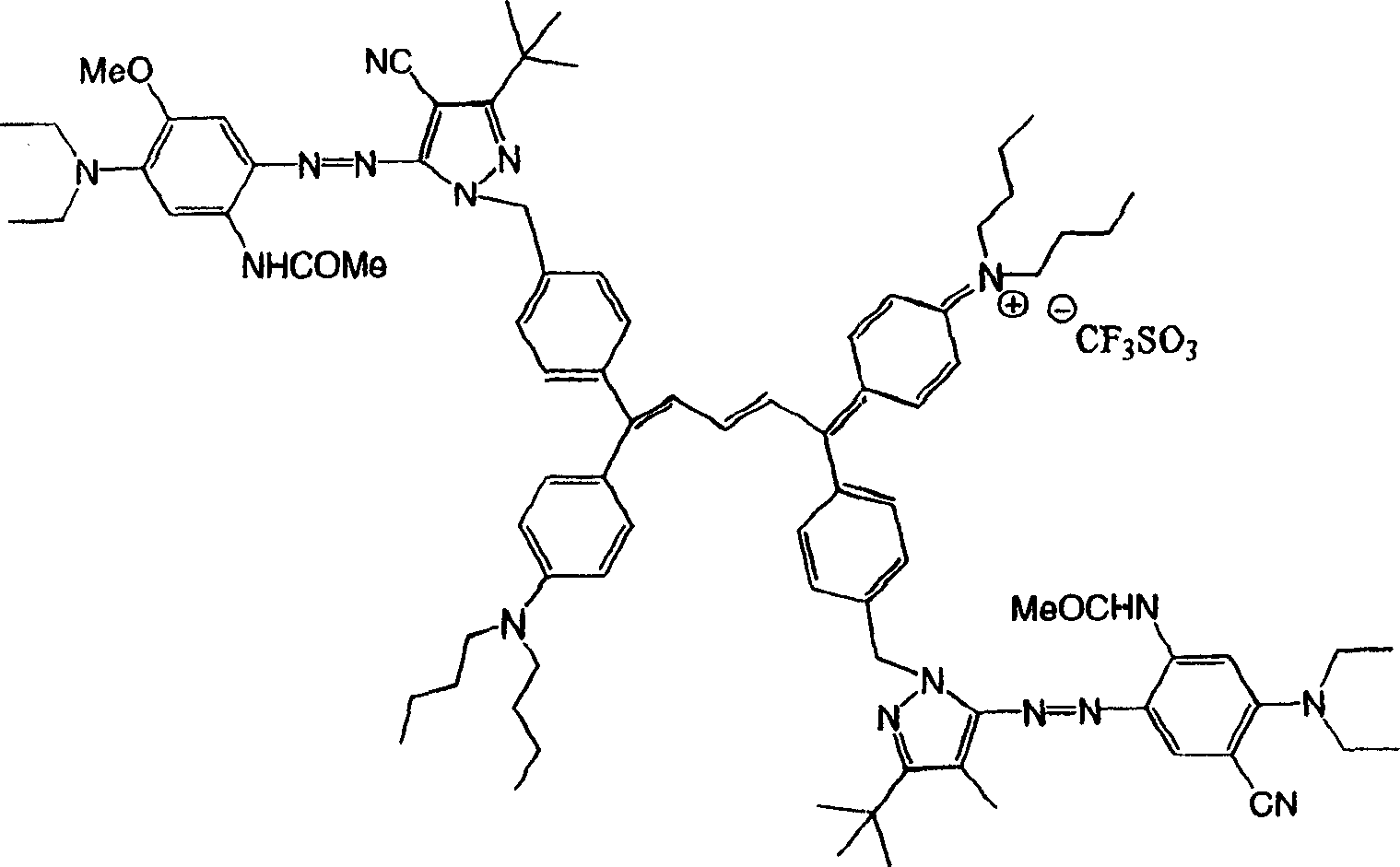
Twin chromophore molecule
A chromophore and molecular technology, applied in the field of twin chromophore molecules, can solve problems such as color pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0031]
[0032] Colorant 1 (λ max1 =420nm;λ max2 =864nm)
[0033]
[0034] Colorant 2 (λ max1 =540nm;λ max2 =575nm;λ max3 =858nm)
[0035]
[0036] Colorant 3 (λ max1 =477nm;λ max2 =862nm)
[0037]
[0038] Colorant 4 (λ max1 =630nm;λ max2 =864nm)
[0039]
[0040] Colorant 5 (λ max1 =380nm;λ max2 =861nm)
[0041]
[0042] Colorant 6 (λ max1 = 400nm; λ max2 =864nm)
[0043]
[0044] Colorant 7 (λ max1 = 460nm; λ max2 =863nm)
[0045]
[0046] Colorant 8 (λ max1 =530nm;λ max2 =865nm)
[0047]
[0048] Colorant 9 (λ max1 =440nm;λ max2 =863nm)
[0049]
[0050] Colorant 10 (λ max1 =530nm;λ max2 =864nm)
[0051]
[0052] Colorant 11 (λ max1 =532nm;λ max2 =861nm)
[0053]
[0054] Colorant 12 (λ max1 =610nm;λ max2 =862nm)
[0055]
[0056] Colorant 13 (λ max1 =63nm;λ max2 =861nm)
[0057]
[0058] Colorant 14 (λ max1 = 450nm; λ max2 =863nm)
[0059]
[0060] Colorant 15 (λ max1 =550nm;λ max2 =867n...
Embodiment
[0087] The IR colorant samples listed below were used as control compounds to illustrate the advantages of the present invention. These control compounds are mixtures (C-1 to C-5) of IR and image colorants (1:2 ratio) or other infrared colorants (C-6 to C-5) that substantially absorb in the visible part of the electromagnetic wave absorption range. 9). Control colorants C-6 to C-8 were obtained by the preparation methods described in US Patent 6,248,886 and US Patent 6,248,893. Colorant C-9 was prepared according to the method described in the published literature (J. Chem. Res. Synpos. (1990), (2), 50-51).
[0088]
[0089]
[0090]
[0091]
[0092] Embodiment 1--photostability
[0093] Element 1 of the invention
[0094] On a poly(ethylene terephthalate) support at 0.1 g / m 2 Apply colorant 1 at 0.5g / m 2 A cellulose acetate propionate binder was applied to prepare the element. The solvent used for coating was a 70 / 30 v / v mixture of methyl isobutyl ketone an...
Embodiment 2
[0112] Example 2 - dark stability (dark stability)
[0113] Example 1 was repeated except that the elements were placed in a dark box with constant dry air flow for periods ranging from 24 hours to 4 weeks.
[0114] Control element CE-6
[0115] This element was identical to Element 1 except that Comparative Colorant Sample C-6 was used in place of Colorant 1.
[0116] Control element CE-7
[0117] This element was identical to Element 1 except that Comparative Colorant Sample C-7 was used in place of Colorant 1.
[0118] Control element CE-8
[0119] This element was identical to Element 1 except that Comparative Colorant Sample C-8 was used in place of Colorant 1.
[0120] Control element CE-9
[0121] This element was identical to Element 1 except that Comparative Colorant Sample C-9 was used in place of Colorant 1.
[0122] All of the stains provided by the invention (stains 1 to 16) showed excellent dark stability; practically no loss in optical density was observed...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com