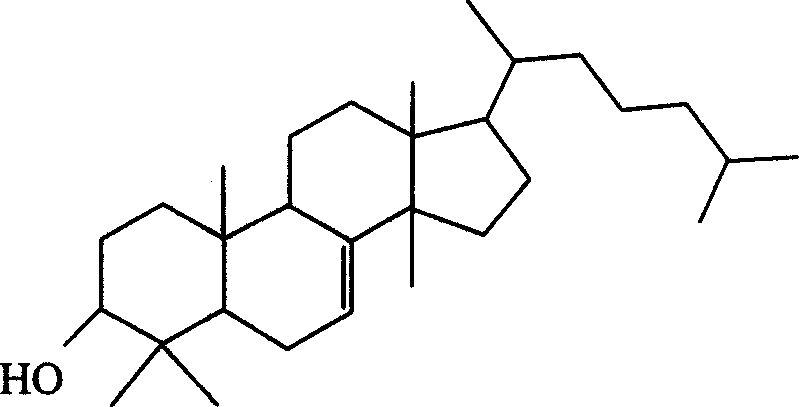
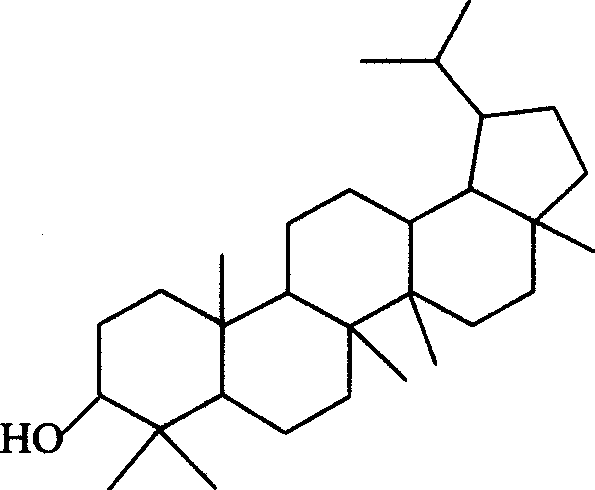
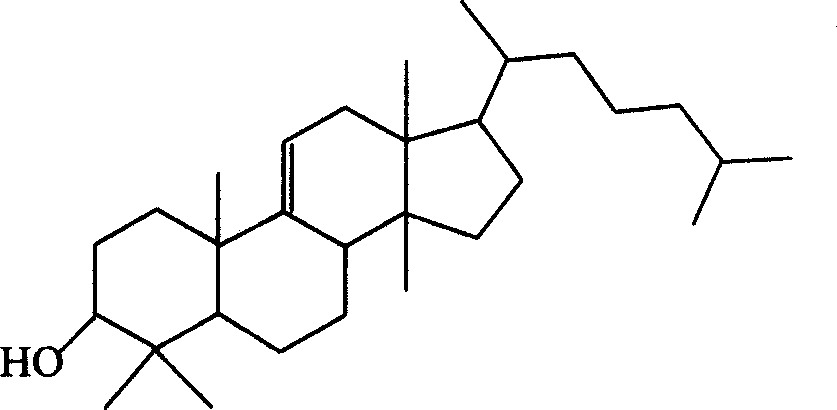
Dihydro-triterpenes in treatment of viral infections, cardiovascular disease, inflammation, hypersensitivity or pain
A technology of triterpene and dihydrogen, which is applied in the direction of cardiovascular system diseases, antiviral agents, and medical raw materials derived from angiosperms, can solve problems such as lack of efficacy, achieve inflammation or pain relief, have no serious side effects, and inhibit Effects of viral infection and inflammation or hypersensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0158] Research overview
[0159] A composition of the present invention comprising 3.1% (w / w) dihydrobutyryl cetyl acetate and 2.8% (w / w) dihydrolupeyl acetate formulated in triglycerides, respectively Its possible antiviral effect against herpes simplex virus and influenza A virus was evaluated in monkey kidney (Vero) cells and MDCK cells (dot test).
[0160] IC of the composition of the present invention at 40-200 μg / ml 50 Both viruses were inhibited at doses.
[0161] test substance
[0162] A composition according to the invention is prepared by hydrogenation and fractionation of Shea Butter (Butyros permum parkii). The composition was analyzed by GC-MS, EI in full scan mode. A HP-5 column 30 m, ID 0.25 mm, 0.25 μm film thickness (5% diphenyl, 95% dimethylpolysiloxane) was used. The sample was dissolved in ethyl acetate (1 mg / mL), and the components were quantified using stigmasterol as an internal standard (0.02 mg / mL in ethyl acetate). The composition was found to...
example 2
[0175] overview
[0176] The composition of the invention described in Example 1 was evaluated for acute oral toxicity in mice. The substance was found to produce no toxicity or mortality at a dose of 2000 mg / kg. Therefore, it can be concluded that the LD 50 Above 2000mg / kg body weight.
[0177] test substance
[0178] The composition of the invention described in Example 1 was used in this experiment.
[0179] research description
[0180] Acute oral toxicity in rats is in accordance with the OECD guideline No 420, "Acute Oral Toxicity-fixed Dose Method", July 1992 and the EEC Directive Published in: "Official Journal of the European Communities" No: L 383A, volume 35, 29.12.1992 , Part B1 "Acute Toxicity (Oral)-Fixed Dose Method" recommended method for determination.
[0181] The study was initiated with a study in which 2000 mg of the composition / kg body weight was administered to one dam. No clinical signs of toxicity were observed in this mouse.
[0182] According...
example 3
[0186] overview
[0187] The composition of the invention described in Example 1 was evaluated for its acute topical anti-inflammatory effect in the phorbolester ear edema test in mice. At the two tested doses, the composition of the present invention was able to significantly suppress ear edema.
[0188] Target
[0189] When it has been confirmed in a separate experiment, the composition of Example 1 of the present invention, under laboratory conditions, is able to inhibit the production of inflammatory cytokines (TNF-α and IL-6) in liposugar-stimulated peritoneal phagocytes (mouse). secretion, we decided to test the efficacy of the composition under natural conditions in the otitis test induced by phorbol acetate (TPA) in mice, which is a commonly used method for screening and Methods for evaluating anti-inflammatory drugs. Locoid(R) infected skin solution (0.1% hydrocortisone 17-butyrate) was used as a positive control.
[0190] Test Articles and Excipients
[0191] Th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com