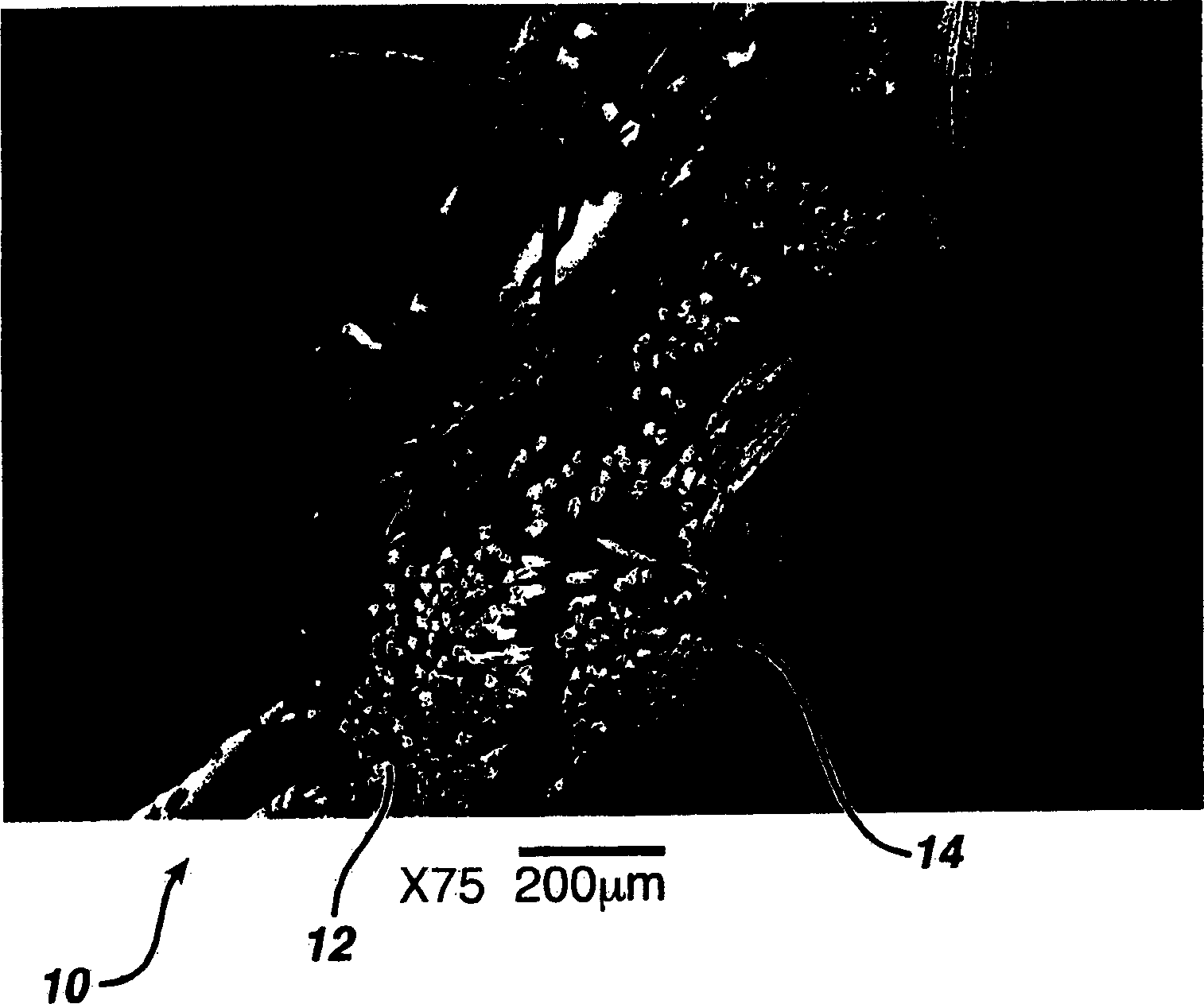
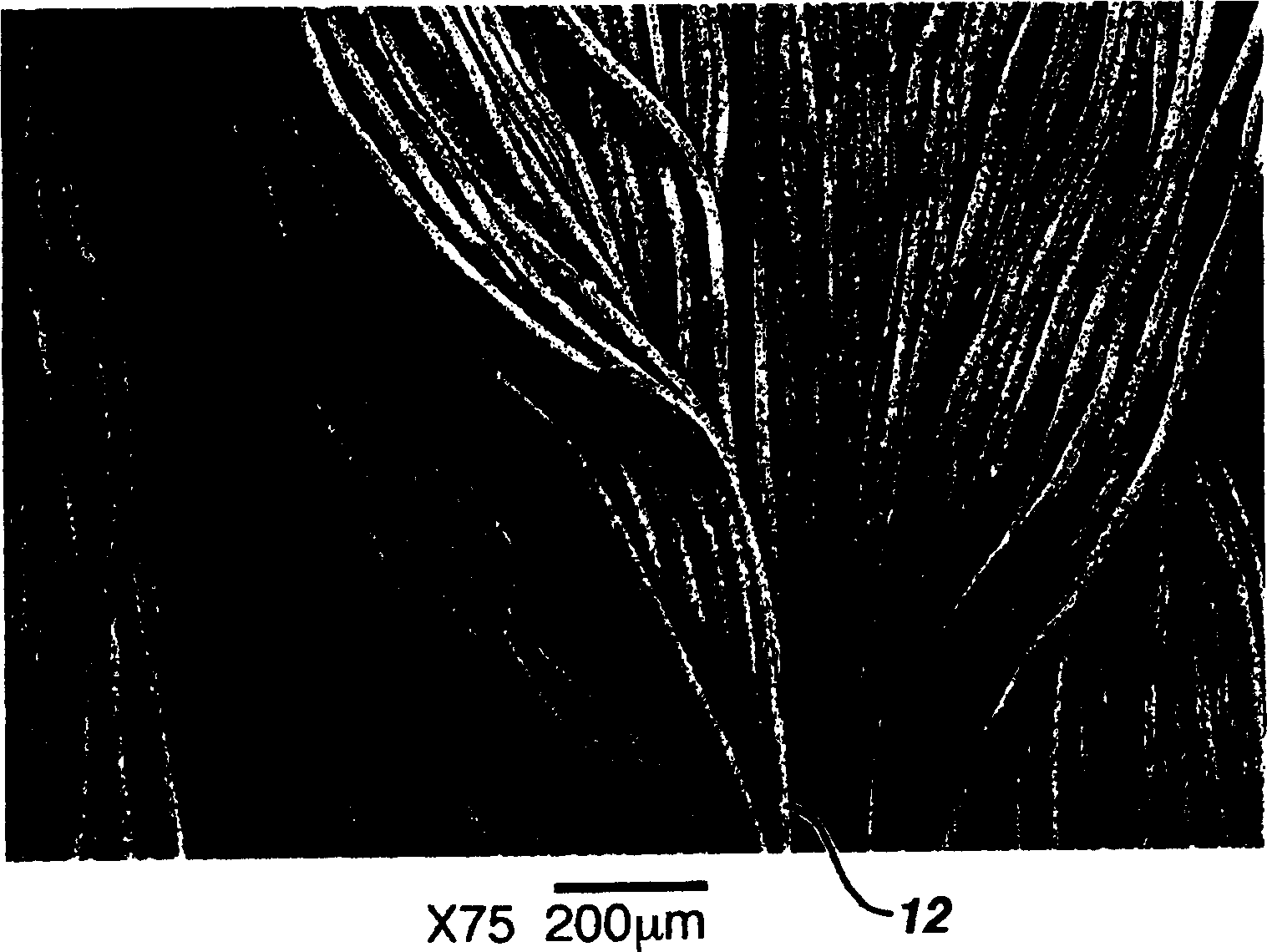
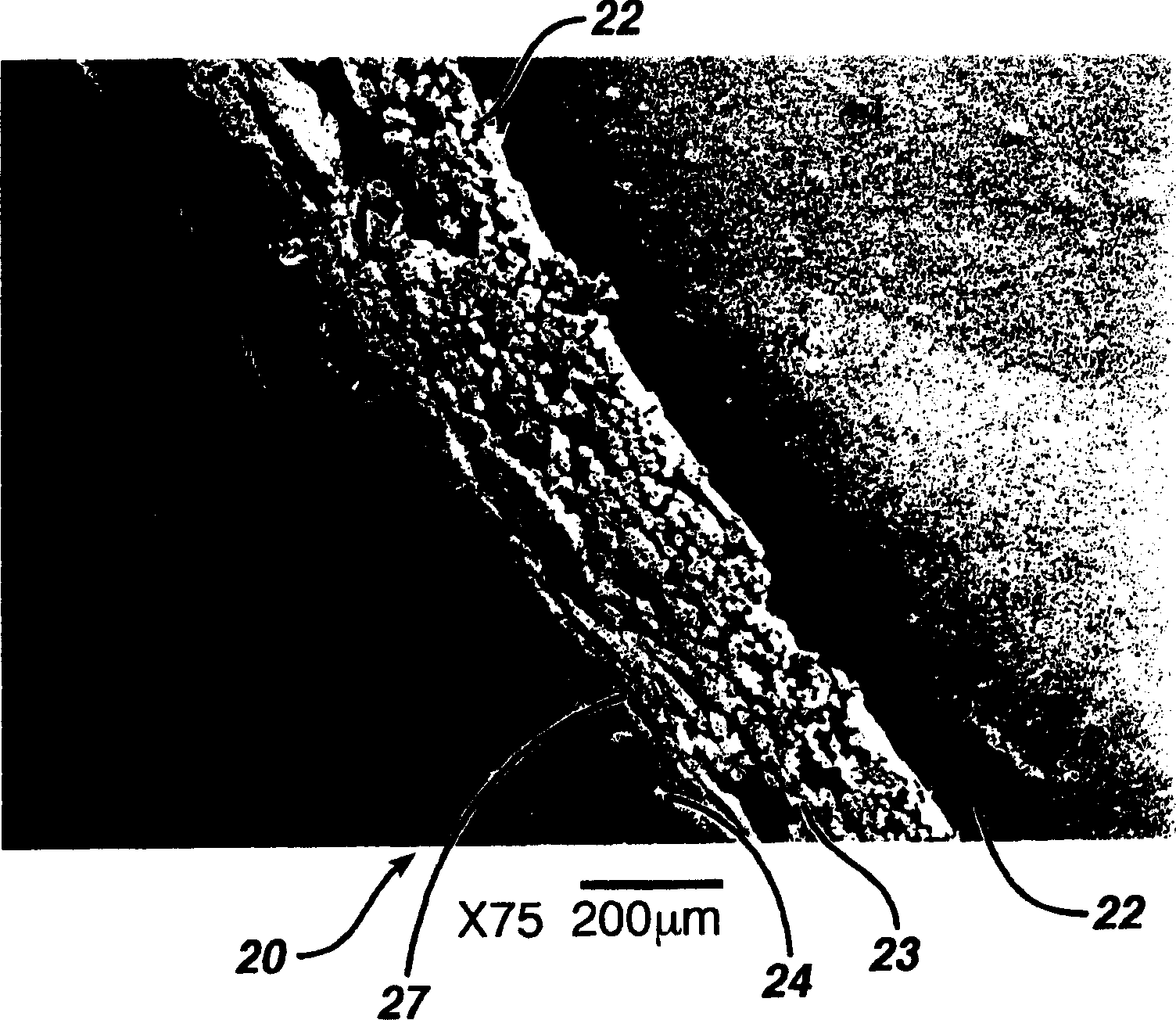
Hemostatic wound dressing and its producing method
A technology for wound dressings and hemostatic agents, which is used in pharmaceutical formulations, other medical devices, knitting, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0136] Preparation of carboxylated oxidized regenerated cellulose (CORC) / HEC porous membrane:
[0137] 1 gram of carboxyethylcellulose (HEC, from Aldrich) was dissolved in 99 grams of deionized water. After the polymer was completely dissolved, 10 grams of the HEC solution was transferred into a crystallization dish with a diameter of 10 cm. A piece of CORC-based Surgicel Nu-Knit(R) absorbable hemostat, 9.8 cm in diameter (approximately 1.3 grams), was placed on top of the HEC solution in the crystallization tray. After soaking the fabric in the solution for 3 minutes, the wet fabric in the tray was then subjected to lyophilization overnight. Forms a very flexible diaphragm. The membrane was further dried under vacuum at room temperature.
Embodiment 2
[0139] Preparation of CORC / CS porous membrane:
[0140] 1 gram of cellulose sulfate (CS, from ACROS Organics) was dissolved in 99 grams of deionized water. After the polymer was completely dissolved, 10 grams of the CS solution was transferred into a crystallization dish with a diameter of 10 cm. A piece of Surgicel Nu-Knit(R) absorbable hemostat, 9.8 cm in diameter (approximately 1.3 grams), was placed on top of the CS solution in the crystallization tray. After soaking the fabric in the solution for 3 minutes, the wet fabric in the tray was then subjected to lyophilization overnight. Forms a very flexible diaphragm. The membrane was further dried under vacuum at room temperature.
Embodiment 3
[0142] Preparation of CORC / MC porous membrane:
[0143] 1 gram of methylcellulose (MC, from Aldrich) was dissolved in 99 grams of deionized water. After the polymer was completely dissolved, 10 grams of the MC solution was transferred into a crystallization dish with a diameter of 10 cm. A piece of Surgicel Nu-Knit(R) absorbable hemostat, 9.8 cm in diameter (approximately 1.3 grams), was placed on the MC solution in the crystallization tray. After soaking the fabric in the solution for 3 minutes, the wet fabric in the tray was then subjected to lyophilization overnight. Forms a very flexible diaphragm. The membrane was further dried under vacuum at room temperature.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com