Tertiary butyl phenol synthesis method by methyl tertiary butyl ether alkylation reaction
A technology of methyl tert-butyl ether and tert-butyl phenol is applied in the field of preparation of tert-butyl phenol series products, which can solve the problems of large loss and industrial application of MTBE method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
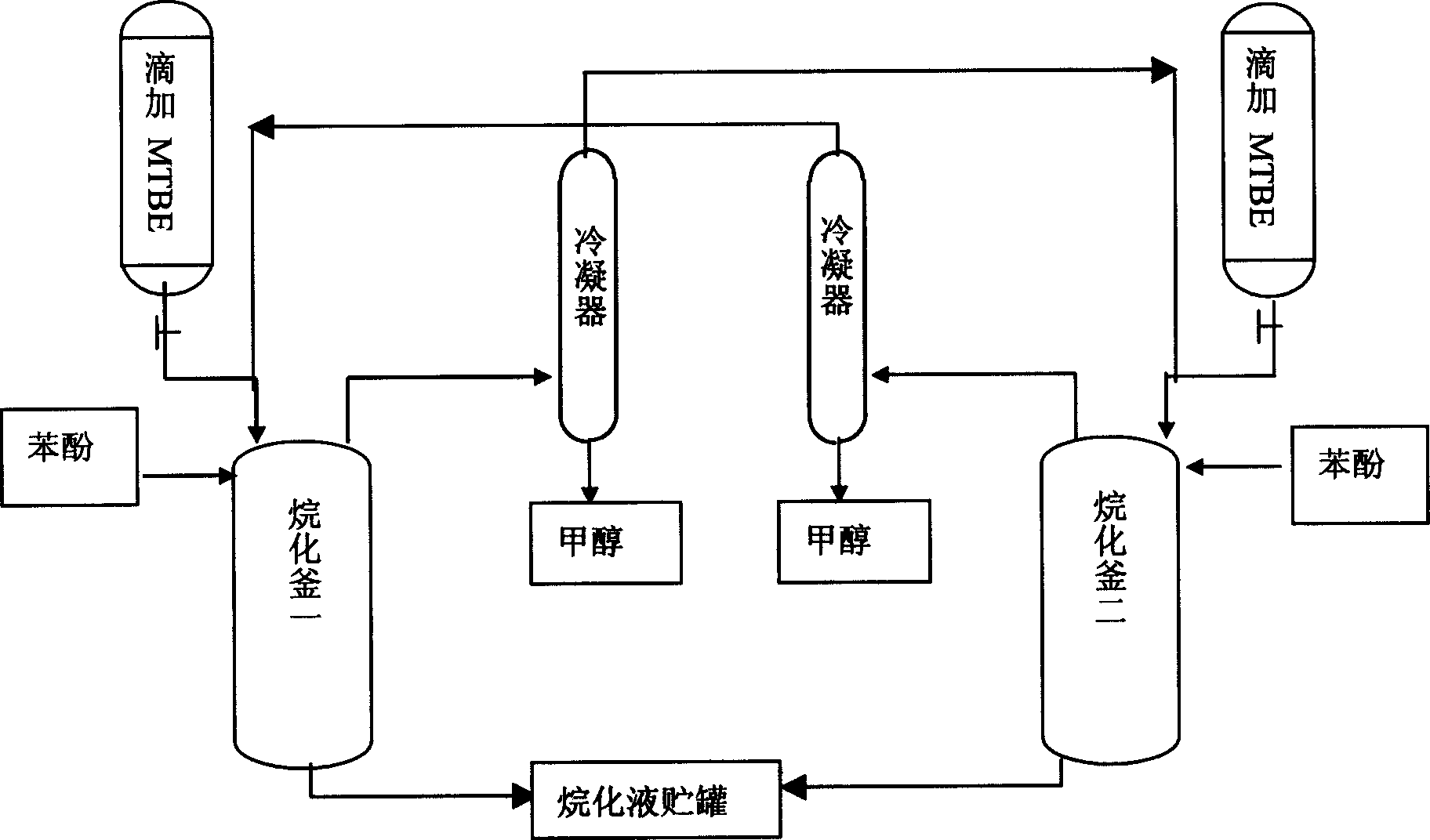
[0015] with attached figure 1 As shown in the double-tank reaction process, add 100g of phenolic reaction raw material phenol and catalyst to reactor 1 and 2, heat up to 80°C, start adding MTBE dropwise to reactor 1, and reactor 2 absorbs reactor 1. The volatilized trace amount of MTBE and the isobutene generated by the cracking are carried out in an alkylation reaction simultaneously. After dripping, sampling and analysis showed that the content of phenol in the alkylation solution was 0.45%, p-tert-butylphenol 18.24%, o-tert-butylphenol 1.36%, 2.6-di-tert-butylphenol 0.34%, 2.4-di-tert-butylphenol 73.58% %, 2.4.6-tri-tert-butylphenol 6.03%, alternately use one kettle to absorb another kettle reaction, after 5 cycles, the MTBE / phenol feeding (mol ratio) is 2.1 / 1 on average, and the alkylation liquid weighs 200g 1. Methanol weighs 90g, the input material is 197g+100g is 297g, and the material loss before and after is 7g.
Embodiment 2
[0019] Using the same double-tank reaction process as in Example 1, add 110 g of reaction raw material phenol and a catalyst in reactor one and two, control the temperature at 70°C to 160°C, and start to add MTBE dropwise in reactor one. The trace amount of MTBE volatilized in the first absorption reactor and the isobutene generated by the cracking are carried out simultaneously for the alkylation reaction. After dripping, sampling and analysis showed 17.35% phenol content, 4.24% o-tert-butylphenol content, 72.51% p-tert-butylphenol content, 0.58% 2.6-di-tert-butylphenol content, and 5.24% 2.4-di-tert-butylphenol content. Alternately use two kettles as the reaction kettle and the absorption kettle. After 5 cycles, the average MTBE / phenol feeding (mol ratio) is 1 / 1.07, and the average weight of each batch of alkylation liquid is 180g, and the weight of methanol is 38g. Add 220g of material, the loss is only 2g before and after.
Embodiment 3
[0023] Using the same double-tank reaction process as in Example 1, 118 g of p-cresol and a catalyst were respectively added to reactors one and two. Control the temperature at 50-100°C, add MTBE dropwise in one kettle, and absorb it in the other kettle, and do 5 batches of experiments. The average amount of MTBE added dropwise is 262ml. Sampling analysis shows that the average content of the alkylating solution is: p-cresol content 1.35%, 2-tert-butyl-4-methylphenol 35.23%, 2.6-di-tert-butyl-4-methylphenol 63.25%, other 0.17%. Alkylation liquid weighs 220g, methanol is 86g, the amount of material added is 312g, and the loss before and after is 6g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com