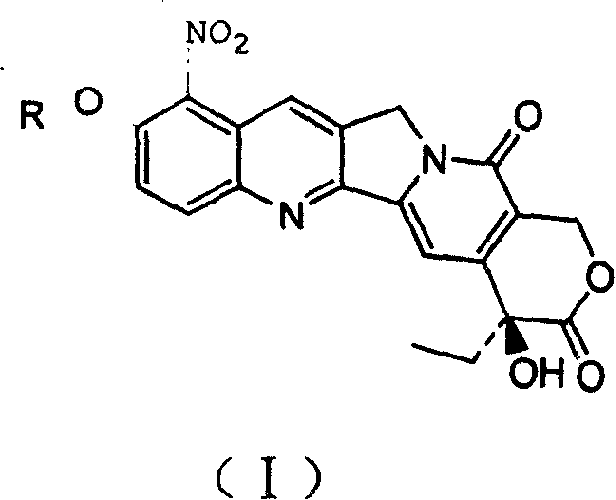
10-alkoxy camptothecine derivative and its preparing method and use
A technology of alkyl and alkane groups, applied to 10-alkoxycamptothecin derivatives and the fields of preparation and application thereof, can solve the problems of difficulty in continuing clinical research, reduced activity in the open-ring form, severe bleeding and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0012] The preparation method of above-mentioned general formula (I) compound has following two kinds:
[0013] method 1
[0014] The preparation method of general formula (I) compound, its step is as follows:
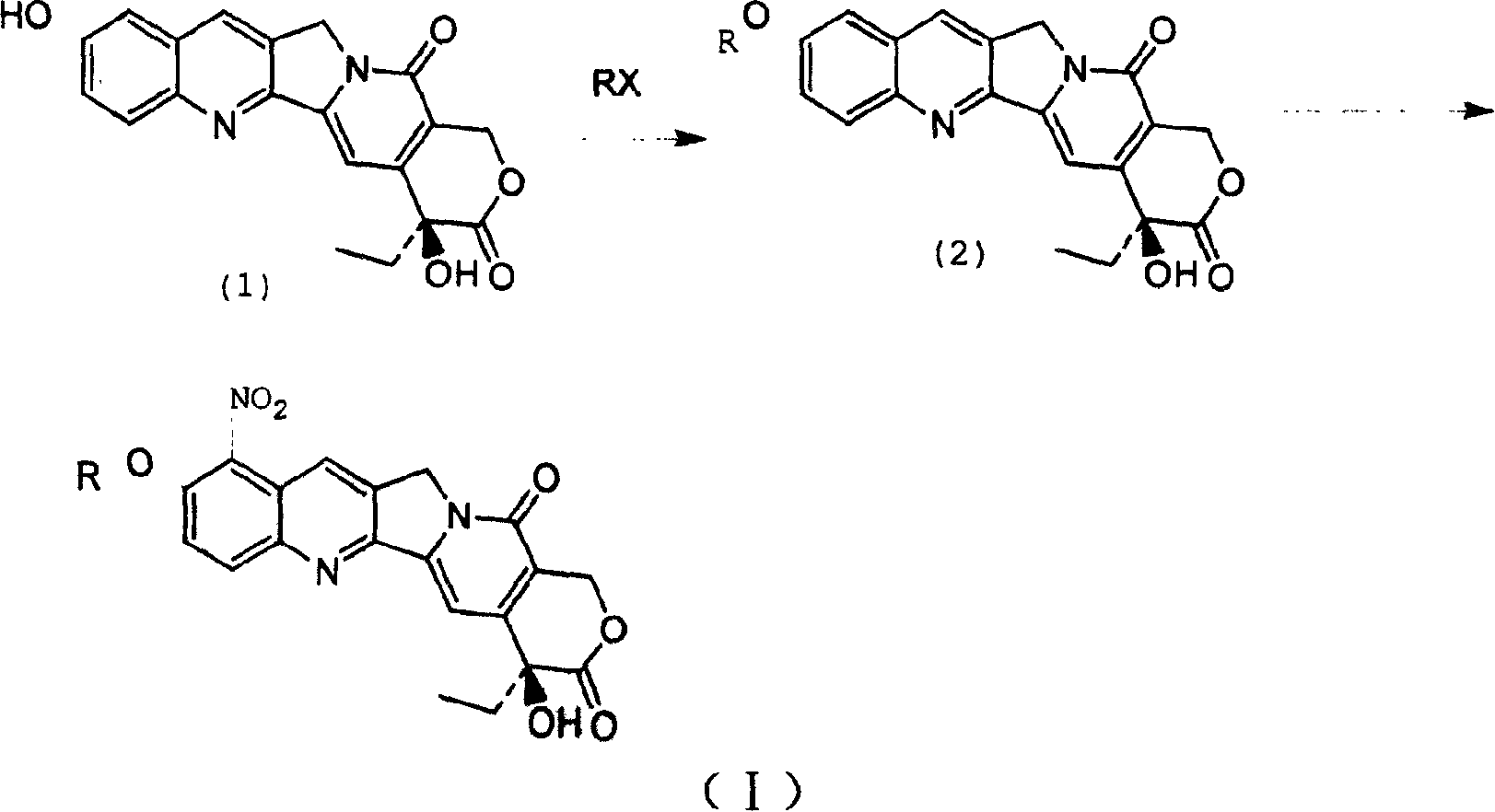
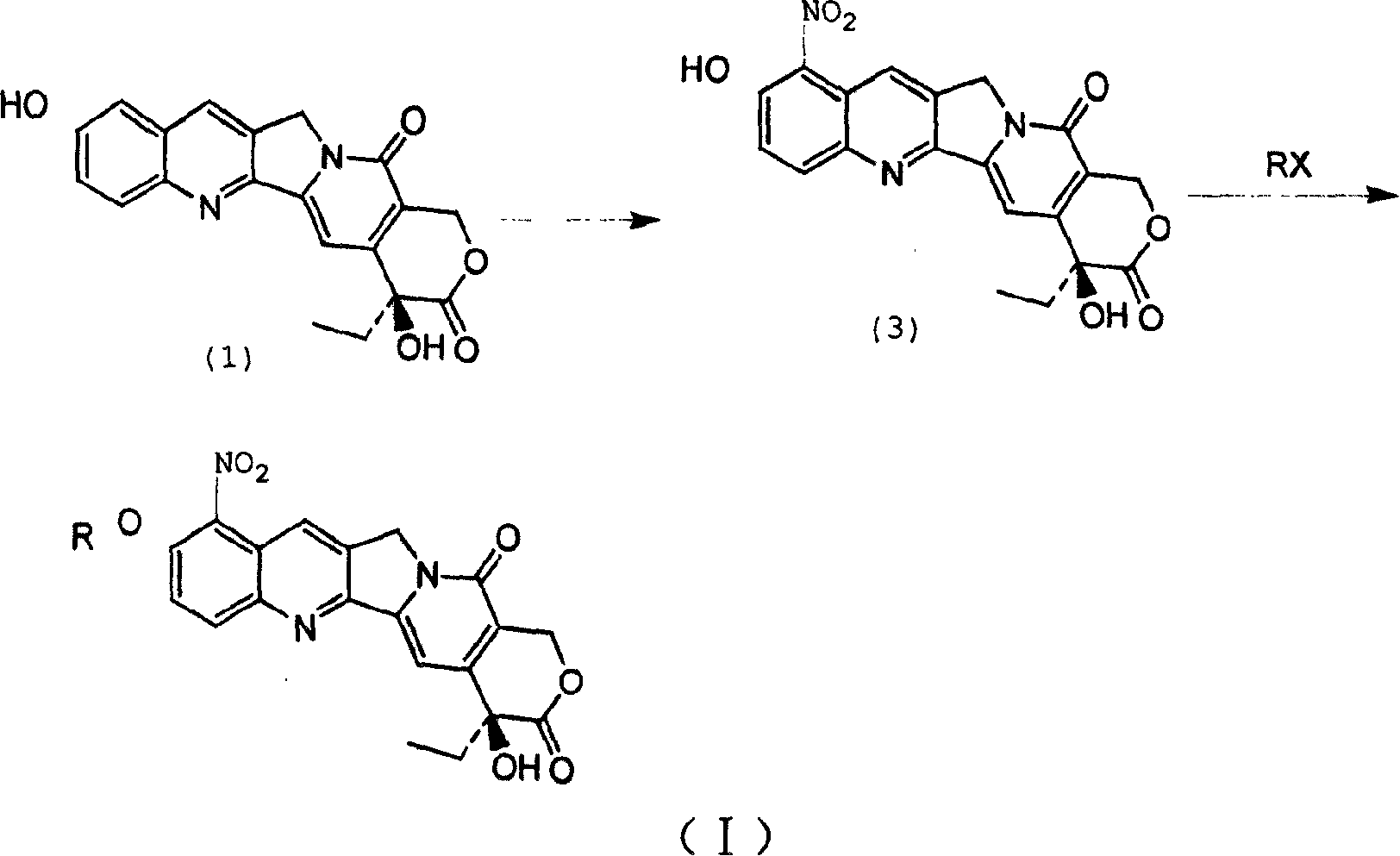
[0015] The hydroxycamptothecin represented by the following formula (1) is reacted with the corresponding RX in a solvent under base catalysis at a temperature of 0-160° C. for 1-50 hours to obtain the intermediate 10 represented by the following formula (2): position etherification, and then carry out nitration at a temperature of -20 to 50°C to obtain a compound of general formula (I), wherein the above-mentioned R is C 1~20 Alkyl, C 1~20 Alkenyl or C 1~20 Hydroxy-containing alkane group, X is Cl, Br and I and CH 3 SO 3 , CF 3 SO 3 ,p-CH 3 C 6 h 4 SO 3 ;
[0016]
[0017] where R represents C 1~20 Alkyl, C 1~20 Alkenyl or C 1~20 Hydroxyl-containing alkane group, X represents Cl, Br and I and CH 3 SO 3 , CF 3 SO 3 ,p-CH 3 C 6 h 4 SO 3 .
[0...
Embodiment 1
[0029] Example 1 10-methoxycamptothecin (MOCPT)
[0030] HCPT (140mg) was completely dissolved in anhydrous DMF (8mL) under heating, and anhydrous K 2 CO 3 (150mg) and CH 3 I (0.09mL), reacted at 80°C for 2.5h, filtered while it was hot, recovered the solvent, added water, neutralized with 10% hydrochloric acid, filtered, and dried to obtain 105mg of 10-methoxycamptothecin, a light yellow solid, with a yield of 75 %, mp248°C.
Embodiment 2
[0031] Example 2 10-methoxy-9-nitrocamptothecin (MONCPT)
[0032] Under ice cooling, 10-methoxycamptothecin (105 mg) was dissolved in concentrated sulfuric acid, and 63% HNO was added 3 (0.02mL), continue to stir for 1.5h, pour the reaction solution into ice water, neutralize with hydrochloric acid, precipitate a solid, filter and dry. Column chromatography gave a yellow product (52 mg), yield 42.1%, mp 245°C (dec.). 1HNMR (δ, ppm, DMSO-d6): 0.86(t, 3H), 1.85(m, 2H), 4.10(s, 3H), 5.25(s, 2H), 5.40(s, 2H), 7.31(s, 1H), 8.03(d, 1H, J=9.6Hz), 8.43(s, 1H), 8.44(d, 1H, J=9.6Hz).EIMS(M + ): 423 (base peak).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com