Medicinal compositions
A technology for compositions and drugs, applied in drug combinations, pharmaceutical formulations, animal/human peptides, etc., and can solve problems such as unclear detailed mechanisms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0160] Experimental example 1 Cell culture, hypoxic stimulation and transient transfection
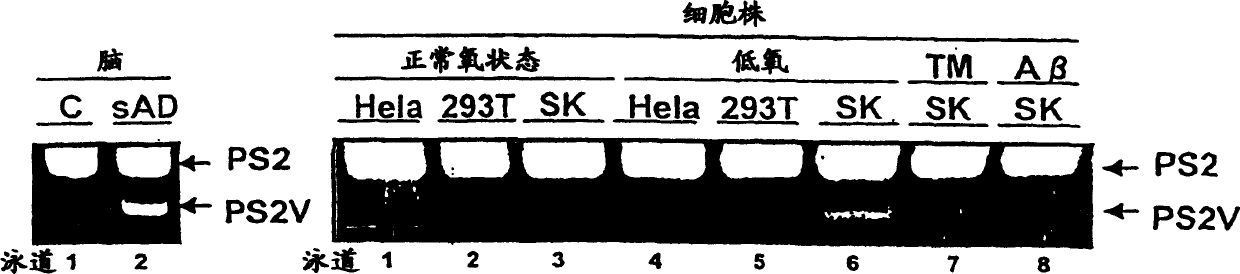
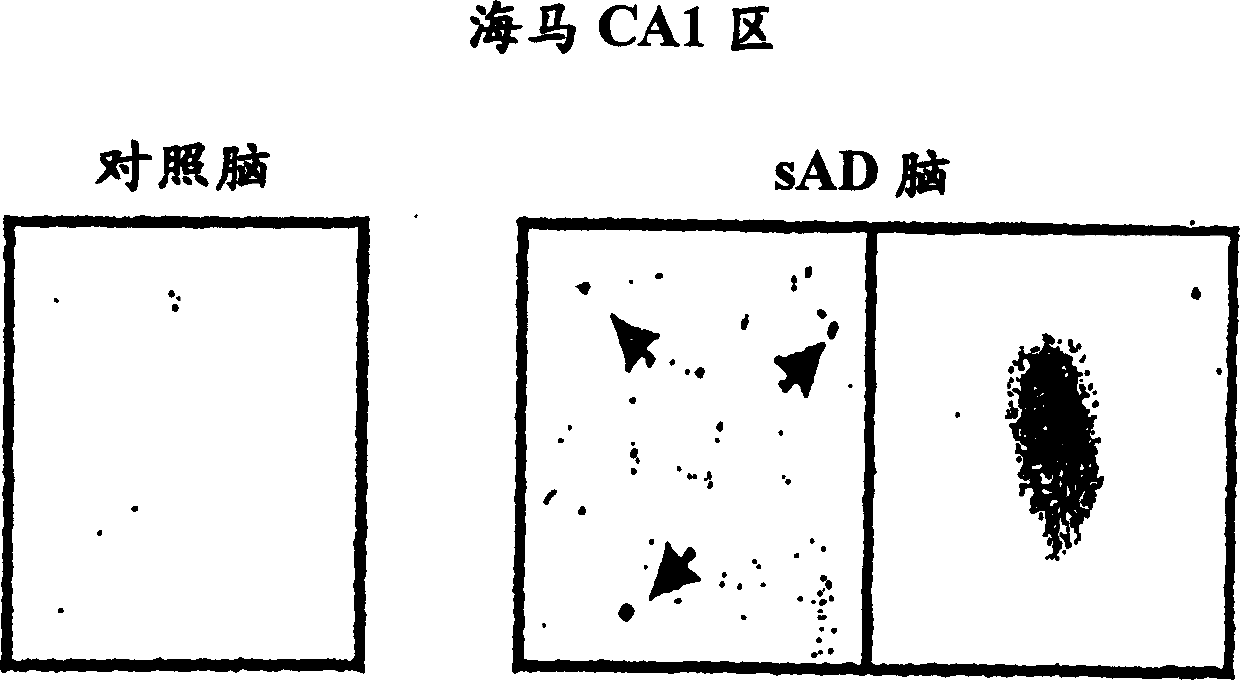
[0161] According to the literature of Sato et al. [Sato N et al., J. Biol. Chem., 276, 2108-2114 (2001)], cell culture and hypoxic stimulation were performed as follows.
[0162] Human neuroblastoma SK-N-SH cells were cultured in αMEM (GIBCO BRL) containing 10% fetal bovine serum in 5% CO 2 , Culture at 37°C. The above cells are at 176.6cm 2 When the dishes were confluent, the cultures were replaced with serum-containing α-MEM for serum-free α-MEM. The medium-changed cultures were incubated for an additional 4 hours.
[0163] HEK-293T cells and HeLa cells were cultured in Dulbecco's minimal medium (GIBCO BRL) containing 10% fetal bovine serum in 5% CO 2 , Culture at 37°C. The above cells are respectively at 176.6cm 2 At confluence in the dish, the culture was replaced with serum-free Dulbecco's minimal medium in the culture. The medium-changed cultures were incubated for an addi...
experiment example 2
[0167] Experimental example 2 Preparation of total RNA and RT-PCR
[0168] According to the literature of Sato et al. [Sato N et al., J.Neurochem., 72, 2498-2505, (1999)], prepare neuroblastoma SK-N-SH cells and HEK-293T cells under various stresses respectively as follows Cell and HeLa cells total RNA, and RT-PCR.
[0169] Using RNeasy total RNA kit (Qiagen), according to the manufacturer's instructions, total RNA was extracted and purified from SK-N-SH cells and HEK-293T cells in normoxia, hypoxia exposure or HMG-I overexpression, respectively. .
[0170]Next, a reverse transcription reaction was performed at 42° C. for 1 hour using the obtained total RNA and mouse Moloney leukemia virus reverse transcriptase (Promega). Nested PCR was then performed using the obtained reaction product as a template. The brief process of PCR is: 95°C for 30 seconds-60°C for 30 seconds-72°C for 1 minute (the final cycle is 2 minutes) as a cycle, a total of 30 cycles. Primer ps251 [5'-attca...
experiment example 3
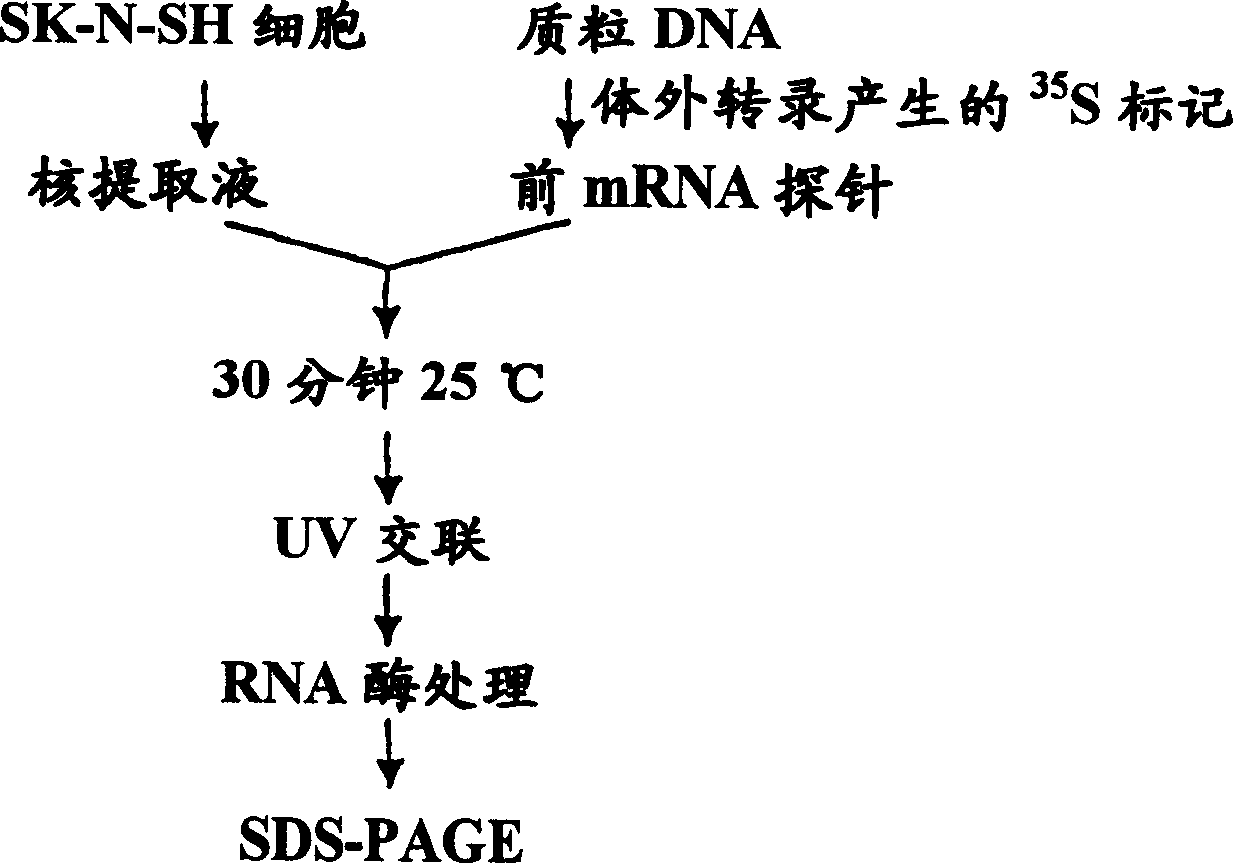
[0172] Experimental Example 3 Preparation of nuclear extract
[0173] According to the modified method of Shreiber et al. [ Yoneda, Y. et al., Neuroscience, 90, 519-533 (1999 )], the nuclear extract was prepared as follows.
[0174] The buffers and other solutions used were filtered and sterilized through Steritop (Milipore) with a pore size of 220 nm before each use.
[0175] Unless otherwise specified, the homogenate of each cell structure substance was mixed with 10mM KCl, 1mM EDTA, 1mM EGTA, 5mM dithiothreitol (DTT) and 1mM (p-amidinophenyl) methanesulfonyl fluoride (PMSF). 50 volumes [315 μl / plate] of 10 mM HEPES-NaOH (pH 7.9) at 2°C.
[0176] Next, 10% ethylphenyl polyethylene glycol (Nonidet P-40) was added to the obtained homogenate so that the final concentration was 0.6%. The resulting mixture was centrifuged at 15000 rpm for 5 minutes. Next, the obtained precipitate was suspended in 10 volumes [0.1 ml] of 20 mM Tris-HCl (pH 7.5) containing 400 mM KCl, 1 mM EDTA, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com